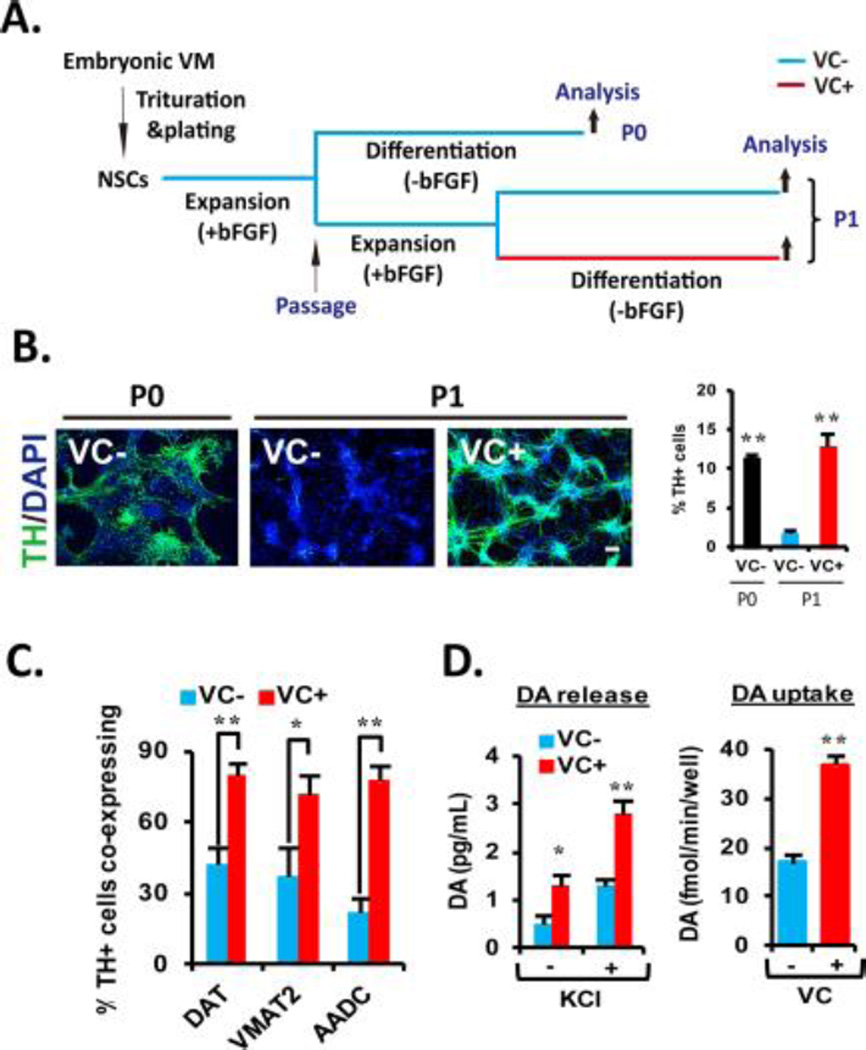
Figure 1. VC enhances the cultured VM-NSC differentiation toward midbrain-type DA neurons equipped with the phenotypical and functional maturities.
(A): Schematic of the experimental design. NSCs derived from rat embryonic VM tissues at E12 were expanded with bFGF for 6 days in vitro, and then followed by 6 days of differentiation in the absence of bFGF (unpassaged, P0). In other case, after P0 expansion, the cultures were passaged, and an additional cell expansion was induced for 4 days (passaged, P1). Differentiation of P1 cultures was induced in the identical conditions with the P0 differentiation. (B): VC treatment rescues cell passage-dependent loss of DA neurogenic potential in VM-NSC cultures. TH+ cell yields were determined in P0 and P1 cultures with or without VC (100 µM). Right: Graph depicting VC treatment effect on percentages of TH+ cells yielded during P1 differentiation. Values represent mean ± SEM; n = 3; *, p < .05 and **, p < .01, one-way ANOVA; this applies to all the experiments hereafter measuring percentage of respective cells. Scale bars are 20 µm in this study. Hereafter, all the following experiments were carried out in P1 cultures with or without VC treatment. (C): VC treatment enhances coexpression of the other DA phenotype genes DAT, VMAT2, and AADC in TH+ cells at D6. (D): Presynaptic DA neuron functions assessed by DA release (left) and uptake (right) assays. The assays were carried out at D16 in the cultures treated or untreated with VC (from D1 to D6). KCl was used to evoke depolarization; n = 4; *, p < .05 and **, p < .01. Abbreviations: bFGF, basic fibroblast growth factor; DA, dopamine; NSC, neural stem cell; TH, tyrosine hydroxylase; VC, vitamin C; VM, ventral midbrain.