Fig. 1.
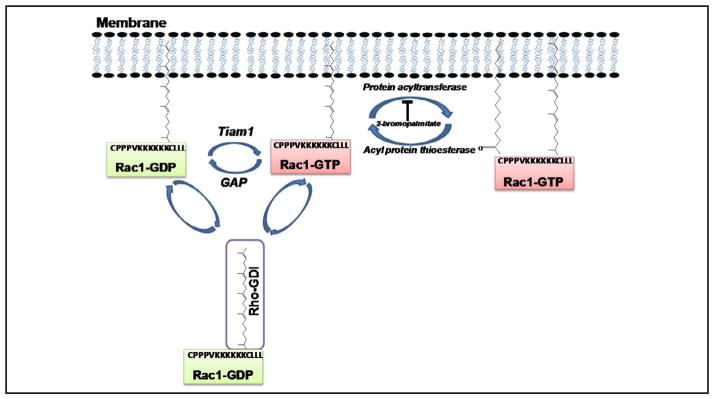
A schematic representation of post-translational modification of Rac1. The majority of small G-proteins (e.g., members of Rho subfamily, Rac1) undergo a series of post-translational modifications at their C-termini, including prenylation and carboxylmethylation [8–11, 46]. In addition, certain G-proteins (Rac1) have also been shown to undergo palmitoylation, catalyzed by protein acyltransferase, at a cysteine residue, which is upstream to the prenylated cysteine. Palmitoylation provides a “firm” anchoring for the modified protein into the cell membrane for optimal interaction with its respective effector proteins [8–11, 46]. Depalmitoylation of these proteins is catalyzed by acyl protein thioesterase. Recent evidence implicates that palmitoylation also promotes Rac1 activation (GTP-bound conformation). Also shown here is activation-deactivation cycle for Rac1. Exchange of GDP for GTP is mediated by Tiam1, a known, guanine nucleotide exchange factor for Rac1. In the current study, we examined putative roles of Tiam1-Rac1 axis (NSC23766) and protein palmitoylation (2-bromopalmitate; 2-BP) in glucose-induced p38 MAP kinase phosphorylation and activation (see text for additional details). Abbreviations used are: Rac1: Ras-related C3 botulinum toxin substrate 1; Rac1-GDP: Rac1 bound to guanosine diphosphate [inactive]; Rac1-GTP: Rac1 bound to guanosine triphosphate; GAP: GTPase activating protein; GDI: guanosine diphosphate dissociation inhibitor; and Tiam1: T-cell lymphoma invasion and metastasis 1.
