FIG 1 .
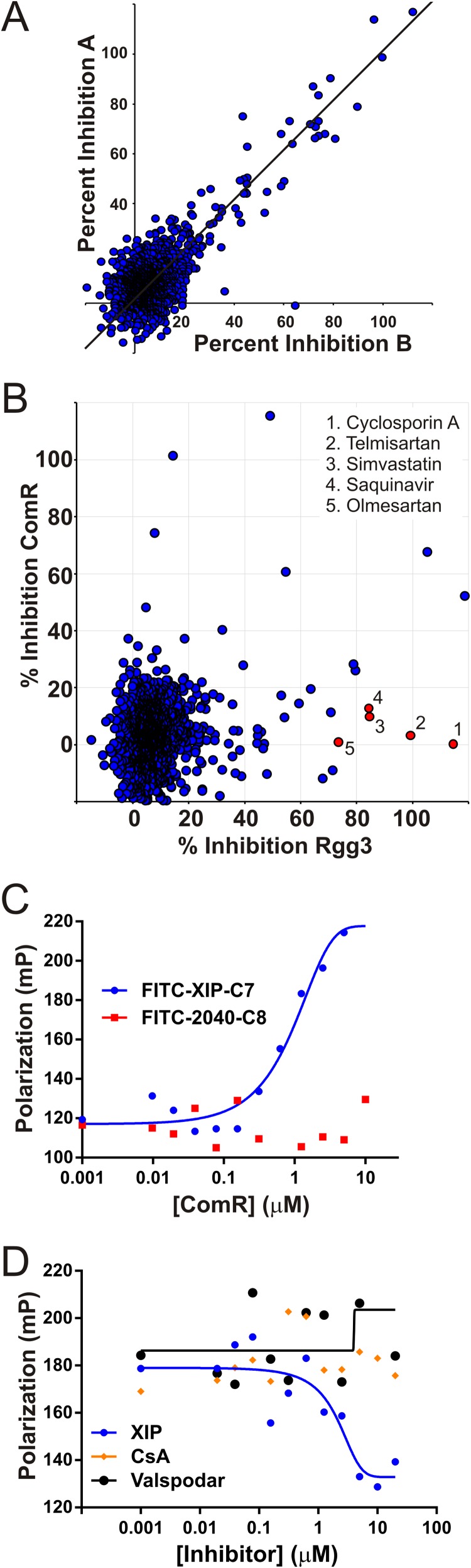
Identification of Rgg3-specific antagonists from high-throughput screening of the drug compound library. (A) Rgg3–FITC-SHP2-C8 complexes were combined with individual compounds of an arrayed drug library and screened in duplicate by fluorescence polarization (FP) to identify those that disrupted the receptor-ligand interaction. Data from two replicate experiments A and B are plotted to illustrate reproducibility. (B) The library was also screened for disruption of ComR–FITC-XIP complexes as was done for Rgg3. Data points denote the percent disruption (i.e., percent inhibition) for each compound using the average of two FP measurements. The top five compounds that selectively inhibited Rgg3 by ≥75% and ComR by ≤20% are highlighted (red dots). (C) FITC-XIP (10 nM) was titrated with purified His-ComR to generate a binding curve by the direct-FP assay. (D) Synthetic unlabeled XIP or HTS compounds were assessed for their ability to compete with FITC-XIP for binding to ComR..