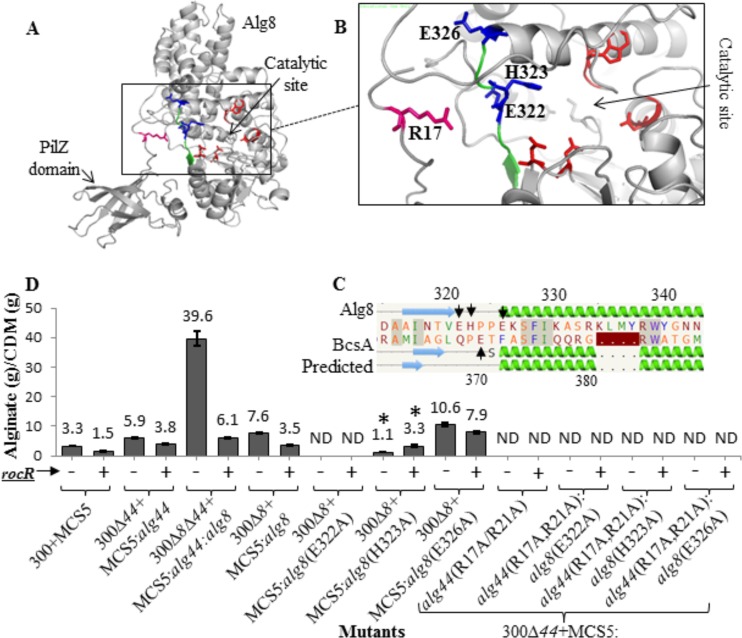
FIG 4 .
Bacterial cellulose synthase-associated autoinhibiting mechanism does not play a role in alginate polymerization. (A) In silico fusion of Alg8-PilZAlg44 was modeled using the Phyre2 server. (B) Highly conserved amino acids (blue sticks; E322, H323, and E326 are labeled with arrows in pairwise alignment of Alg8 with BcsA [C]) were identified in a similar position to that of BcsA and could form a salt bridge with R residues of the PilZAlg44 domain R17XXXR21 motif and were chosen to be replaced by alanine using site-specific mutagenesis. (D) Alginate quantification of PDO300Δalg8, PDO300Δalg44, and PDO300Δalg8Δalg44 transformants with plasmids harboring respective site-specific mutants of alg8 and alg44 with (+) and without (−) the rocR gene. Alg8’s mutated residue which positively responded to c-di-GMP level alteration by RocR is labeled with an asterisk. 300, PDO300; ND, not detectable; MCS5, pBBR1MCS-5.