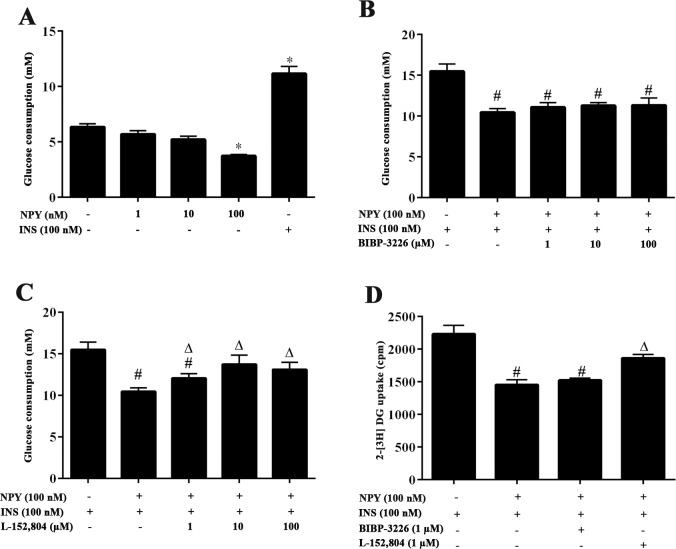
Fig 5. NPY inhibits glucose consumption and 2-[3H] DG uptake in 3T3-L1 adipocytes via the NPY Y5 receptor.
(A) The effect of NPY on basal glucose consumption of 3T3-L1 adipocytes. The differentiated 3T3-L1 adipocytes were incubated with DMEM containing 0.2% BSA for 12 h and then treated with 1–100 nM NPY for 12 h or 100 nM insulin (INS) for 2 h to detect basal glucose consumption. Insulin was used as a positive control. The amount of glucose that disappeared in the medium was determined as the amount of glucose consumption. (B) The effect of Y1 receptor antagonist BIBP-3226 on insulin-stimulated glucose consumption in 3T3-L1 adipocytes treated with NPY. Cells were pre-treated with 1–100 μM BIBP-3226 for 8 h, then subsequently treated with 100 nM NPY for 12 h with 100 nM insulin for 2 h to detect insulin-stimulated glucose consumption. (C) The effect of Y5 receptor antagonist L-152,804 (1–100 μM) on insulin-stimulated glucose consumption of 3T3-L1 adipocytes treated with NPY. The cells were treated as described above. Data are presented as means ± SEM, n = 6; *P<0.5 vs. basal; # P<0.05 vs. INS alone; ∆ P<0.5 vs. NPY+INS. (D) The effect of Y1 receptor antagonist BIBP-3226 and Y5 receptor antagonist L-152,804 on insulin-stimulated 2-[3H] DG uptake in 3T3-L1 adipocytes treated with NPY. The differentiated 3T3-L1 adipocytes were pre-treated with 1 μM L-152,804 or 1 μM BIBP-3226 for 8 h and then treated with 100 nM NPY for 12 h and 100 nM insulin for 20 min in KRH buffer. The cells were subsequently incubated with 0.1 mM 2-deoxy-D-glucose (2-DG) and 0.5 μCi/ml 2-[3H] DG for 10 min. Radioactivity of 2-[3H] DG of the whole cell lysates was measured. Data are presented as means ± SEM, n = 3; # P<0.05 vs. INS alone; ∆ P<0.5 vs. NPY +INS.