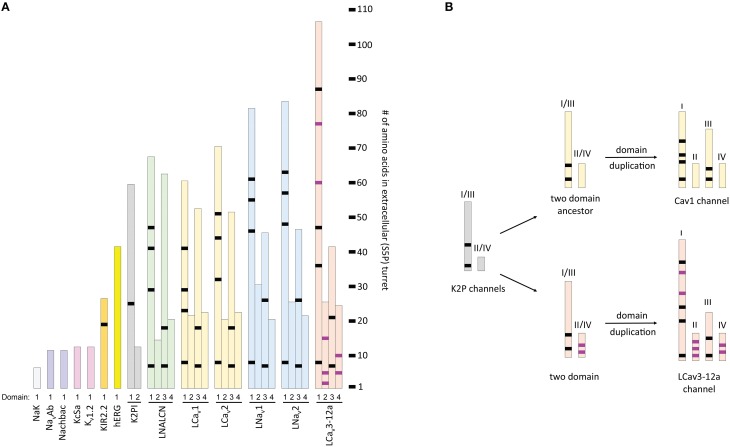
Figure 11.
Relative length of extracellular loops in K channels, two-pore K2P1 channel, and representative 4x6TM channels from the giant pond snail, Lymnaea stagnalis. Locations of shared cysteines are shown in black, while the T-type channel specific cysteines are shown in purple. (A) The size of extracellular turrets varies from 6 to 12 residues in bacterial K and Na channels (NaK, NavAb, NaChBac and KcsA) and Shaker-type voltage-gated K channel (Kv1.2). Longer turrets (26 residues) in the human inward rectifying potassium channel, Kir2.2, change the external landscape of the outer vestibule. Human hERG (Kv11.1) channel has a characteristic amphipathic helix in its extracellular loop (41 residues) that regulates both gating kinetics and ion selectivity. Domain D1 turret in of the K2P channel is much longer (60 residues) than domain D2 turret (12 residues). The long D1-D1 turrets form a helical cap above the pore, bridged by a disulphide bond (Brohawn et al., 2012; Miller and Long, 2012). (B) The long-short-long-short configuration of loops in repeat domains DI-DII-DIII-DIV of 4x6TM channels resembles that in the two domain K2P channel, after undergoing a domain duplication (see inset). Evidence for a kinship between DI-DIII and DII-DIV loops pairs is the conservation of the six core S5P cysteines within all 4x6TM channels in the longer DI-S5P:DIIIS5P pair. T-type channels differ from other 4x6TM channels by having a longer DI loops with additional cysteines and presence of T-type channel specific cysteines in the DII-S5P: DIV-S5P pair.