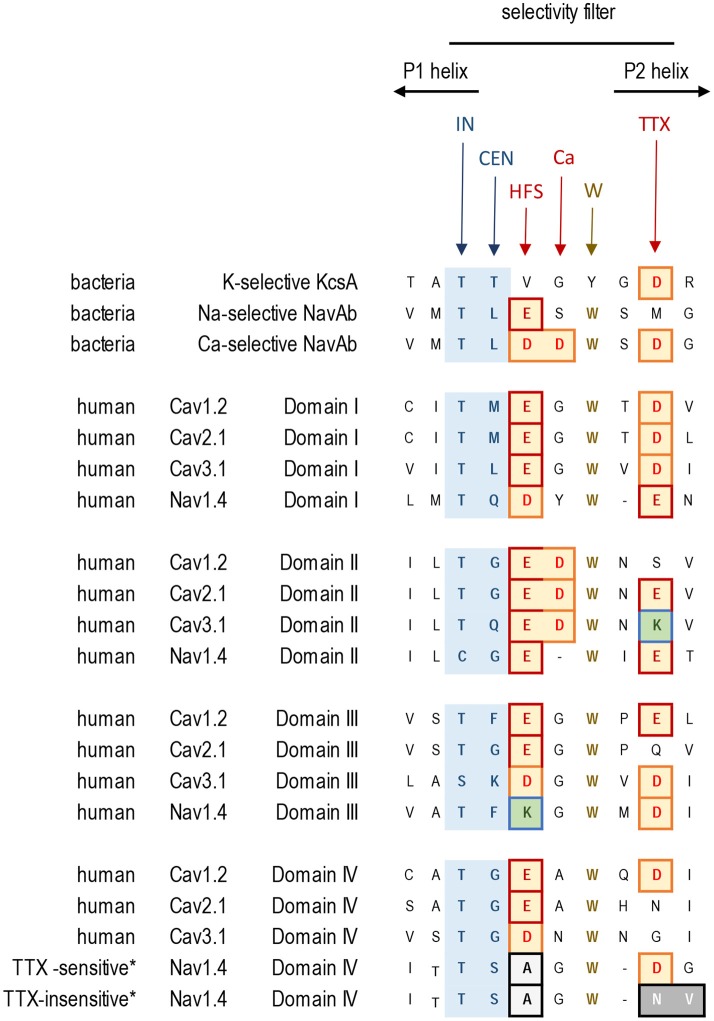
Figure 4.
Alignment of sequences contributing to the selectivity filters in K+, Ca2+, and Na+ channels. Residues contributing to the central (CEN) and inner (IN) sites at the selectivity filter region are highlighted blue. Negatively charged residues contributing to the HFS sites and to the rings of outer carboxylates are red/brown. The aspartate residue in Domain II, which is next to the HFS site, is conserved in Cav1, Cav2, and Cav3 channels. Exceptionally conserved tryptophans form inter-repeat hydrogen bonds that stabilize the P-loop folding. Note the difference in Domain IV of the TTX-sensitive (hNav1.4) and TTX-low-sensitive channels from garter snake (Thamnophis sirtalis) that adapted to feed on TTX-ladened newts by neutralizing a negative charge in the TTX site.