Fig. 7.
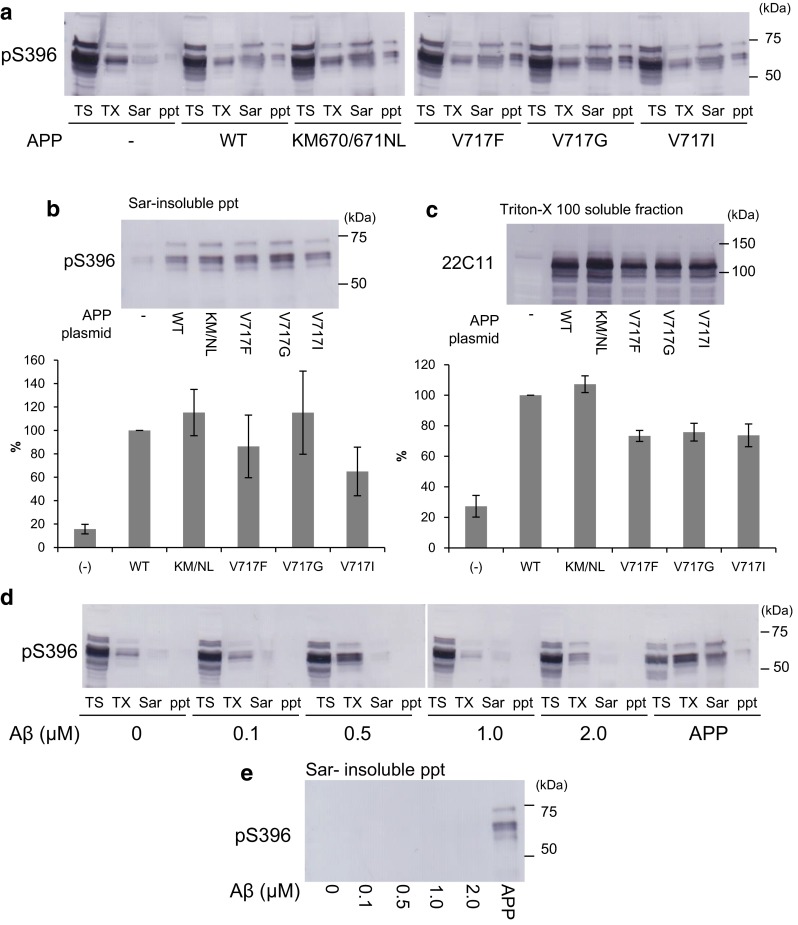
The effect of APP pathogenic mutant and amyloid β on intracellular tau aggregation. Immunoblot analysis of lysates from cells transfected with 4R1N tau, cells transfected with both 4R1N tau and WT-APP, cells transfected with both 4R1N tau and APP KM670/671NL, cells transfected with both 4R1N tau and APP V717F, cells transfected with both 4R1N tau and APP V717G, and cells transfected with both 4R1N tau and APP V717I. All cells were treated with 4R1N tau fibrils (a). The Triton X-100 soluble fraction detected by 22C11 (b) and the Sarkosyl-insoluble pellet fraction detected by pS396 (c) are shown. The results are expressed as means +SE (n = 3). WT-APP was taken as 100 %. Immunoblot analysis of lysates from cells transfected with 4R1N tau and treated with amyloid β (0–2 μM), and cells transfected with both 4R1N tau and APP. All cells were treated with 4R1N tau fibrils (d). The Sarkosyl-insoluble pellet fraction is shown (e). Cells were extracted successively to obtain Tris–HCl-soluble fraction (TS), Triton X-100-soluble fraction (TX), and Sarkosyl-soluble fraction (Sar), leaving the pellet fraction (ppt). Detection was done with pS396
