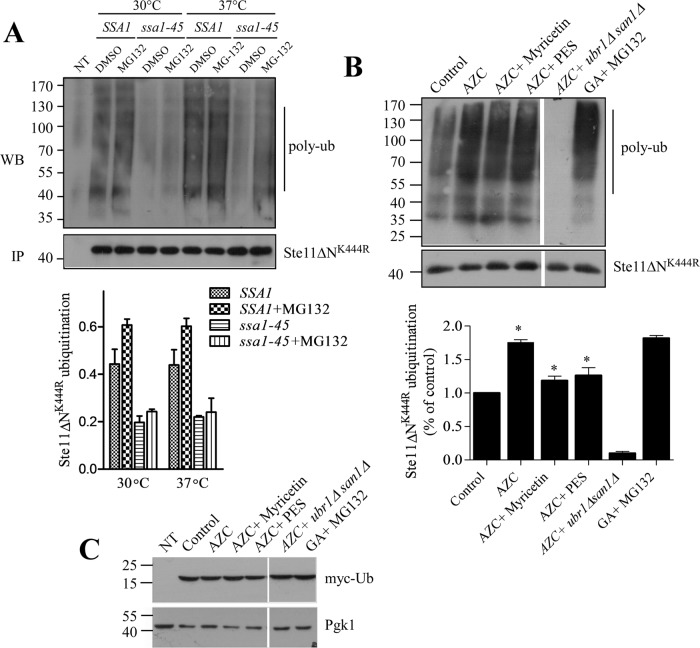
FIGURE 3:
Hsp70 promotes ubiquitylation of kinases for their degradation. (A) Ubiquitylation of Ste11ΔNK444R was assayed in SSA1 and ssa1-45 cells at permissive (30°C) and nonpermissive (37°C) temperatures. MG-132 (100 μM) was added for 2 h to block the proteasome. Ste11ΔNK444R was immunoprecipitated from these cells with anti-His antibody. Ubiquitylated Ste11ΔNK444R was visualized by Western blotting followed by immunostaining with antiubiquitin antibody. Five microliters of immunoprecipitated Ste11ΔNK444R from each sample was loaded onto an SDS–PAGE, and the kinase level was identified by Western blotting probed with anti-His antibody. Vector control (NT) was used to visualize any nonspecific ubiquitination. Ubiquitinated Ste11ΔNK444R was quantified and represented in the graph. (n = 3, bar = ±SE). (B) Ubiquitination of Ste11ΔNK444R kinase was checked after WT yeast cells were treated with the proline analogue AZC (50 mM) alone or in combination with myricetin (100 μM) or PES (150 μM) for 2 h. In this case, Myc-tag ubiquitin was expressed from the CUP promoter after induction with 100 μM of copper. The Ste11ΔNK444R was immunoprecipitated, and polyubiquitylation of kinase was identified by the anti-myc antibody. AZC-induced Ste11ΔNK444R ubiquitylation was checked in the ubiquitin ligases of ubr1Δsan1Δ knockout yeast cells. GA (50 μM)-induced Ste11ΔNK444R ubiquitylation in the presence of MG132 (100 μM) was used as a control. The polyubiquitylation of Ste11ΔNK444R was quantified and represented in the associated graph (n = 3, bar = ± SE). * indicates the significance of p ≤ 0.05. (C) Western blotting with anti-myc antibody showed the same level of myc-ub expression in the yeast cell lysates used for Ste11ΔNK444R ubiquination in B. Vector control (NT) was used for nonspecific interaction with the antibody. Pgk1 served as a loading control.