FIGURE 1:
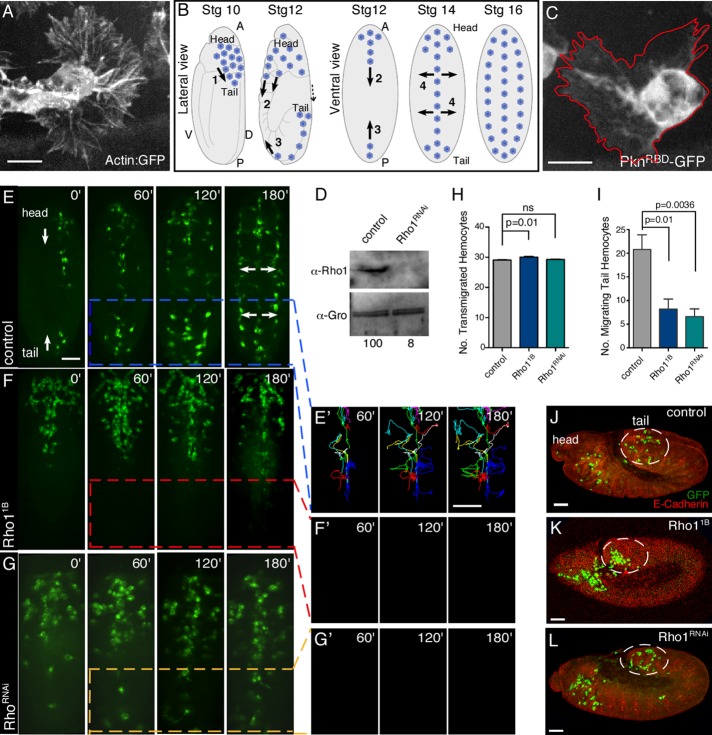
Rho1 participates in a subset of hemocyte developmental migrations. (A) Confocal surface projection micrograph of an otherwise wild-type hemocyte expressing the Actin5C-GFP reporter. (B) Schematic of Drosophila hemocyte developmental migrations in stage 10–16 embryos from lateral or ventral perspectives. The major events indicated are 1) transmigration of hemocytes from the head to the tail, 2) posterior migration of head hemocytes along ventral midline, 3) anterior migration of tail hemocytes along ventral midline, and 4) lateral migration of hemocytes from the ventral midline to form three parallel lines. Head and tail are indicated. A, anterior; P, posterior; D, dorsal; V, ventral. (C) Confocal surface projection micrograph of an otherwise wild-type hemocyte expressing a Rho1 biosensor (UAS-PknRBD-GFP) under the control of the Pxn-Gal4 driver. (D) Western analysis of protein extracts from control (Pxn-Gal4) and Rho1RNAi hemocytes probed with antibodies recognizing Rho1 (α-Rho1(P1D9)) and a loading control (α-Gro). The normalized levels of Rho1 are indicated (with control set to 100). (E–G′) Time-lapse series of surface projections (ventral view) of migrating hemocytes expressing GFP in control (Pxn-Gal4) (E), Rho11B (F), and Rho1RNAi (G) embryos. Head and tail are indicated (E). Hemocytes that began their developmental migrations in the tail were tracked for 180 min (E′, F′, G′). Note that all of the tail hemocytes present in G arrived from the anterior. (H, I) Quantification of the number of transmigrated tail hemocytes (H) and number of these tail hemocytes that migrate anteriorly (I) in control (Pxn-Gal4), Rho11B, and Rho1RNAi embryos (n ≥ 20). (J–L) Confocal surface projection (lateral view) micrographs of stage 10 control (Pxn-Gal4) (J), Rho11B (K), and Rho1RNAi (L) embryos stained for DE-cadherin (red) and GFP (green). Transmigrated hemocytes are circled. All results are given as means ± SEM; p values are indicated. Scale bars, 10 μm (A, C), 40 μm (E–G′, J–L).