FIGURE 7:
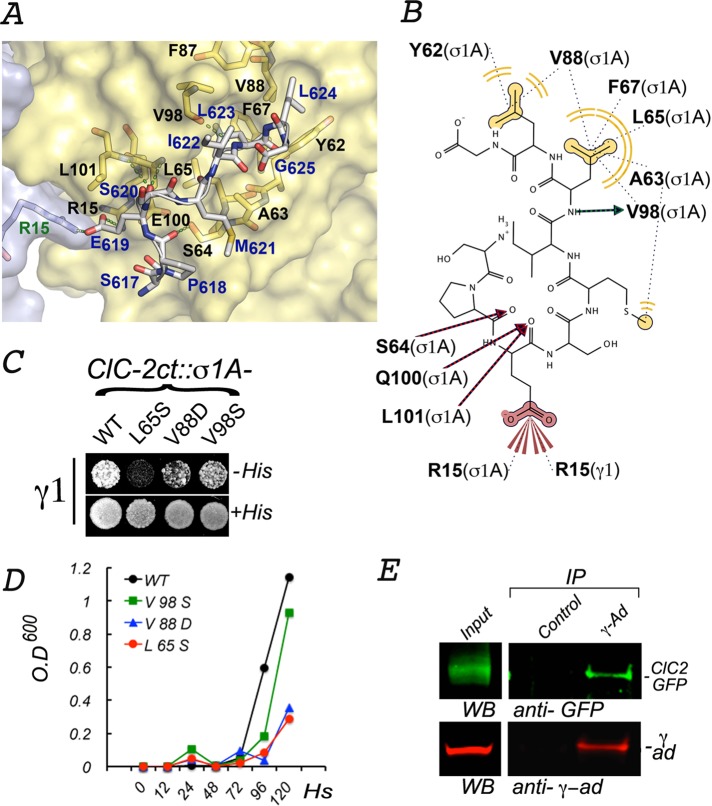
Interactions between ESMI623LL and a conserved pocket in γ1-σ1A. (A) In silico model of the interaction between ESMI623LL and γ1-σ1A. The structure of the γ1-σ1A pocket of AP-1 that interacts with ESMI623LL was modeled from the crystal structure of the corresponding pocket in the α−σ2 hemicomplex of AP-2 (2JKR) (see Materials and Methods). The names of the key pocket residues in the surface-rendered yellow representation of σ1A (black font) and gray representation of γ1 (green font) are shown as yellow and orange sticks. The residues in ESMI623LL (blue font), superimposed on the pocket, are shown in gray sticks with oxygen atoms in red and nitrogen atoms in blue. (B) Two-dimensional representation of the interaction of ESMI623LL with the γ1-σ1A pocket. The model shows the leucine residues in ESMI623LL coordinating with σ1 hydrophobic residues (62Y, 65L, 67Phe, 88V, and 98V), whereas the negatively charged residue 619E at −4 position coordinates with positively charged residues 15R in σ1 and 15R in γ1. (C, D) Yeast three-hybrid assay shows that interaction of CLC-2ct with γ1-σ1A is inhibited by mutations of two of the residues predicted to be involved in the interaction (65L, 88V). (E) Coimmunoprecipitation of ClC-2-GFP and AP-1. On immunoprecipitation with mouse γ-adaptin antibodies, the presence of both ClC-2-GFP and γ-adaptin in the precipitate is observed by Western blot.