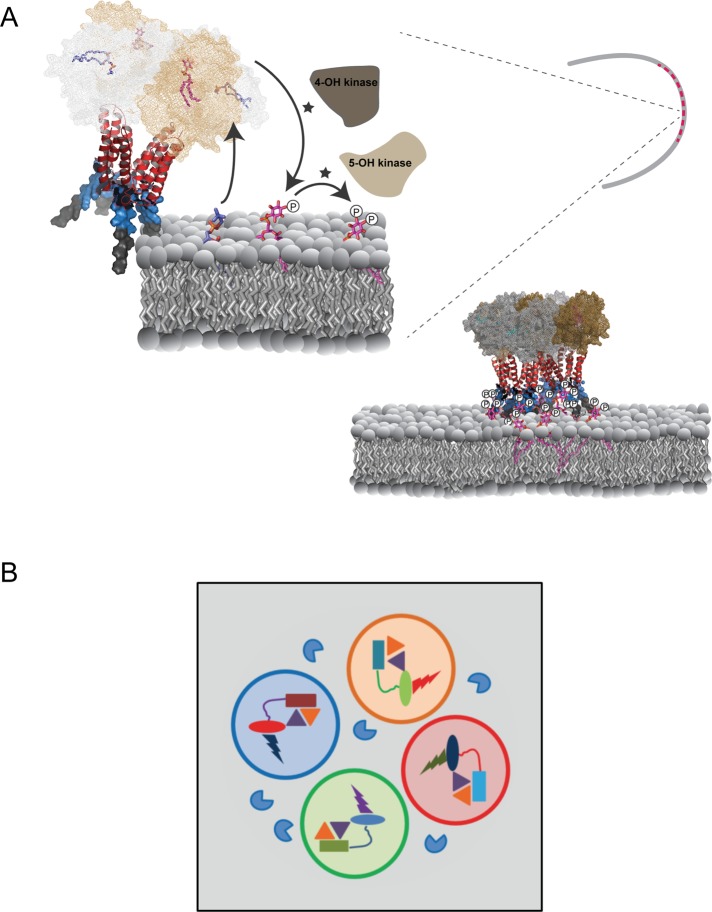
FIGURE 10:
Patterning of phosphoinositide signaling by Sec14-nodulins. (A) AtSfh1-dependent patterning of PtdIns(4,5)P2 in root hair tips. On the basis of the mechanism for how yeast Sec14 stimulates PtdIns 4-OH kinase activity (Schaaf et al., 2008) and our data with the AtSfh1 Sec14 domain (unpublished data), we propose that Sec14 domains (mesh) of an AtSfh1 tetramer promote phosphoinositide synthesis by presentation of PtdIns (magenta) to PtdIns 4-OH kinases during heterotypic lipid exchange with amino phospholipid (blue). Specific interaction of C-terminal lysines (blue surface mode) with PtdIns(4,5)P2, in conjunction with the weak association of the AtSfh1 C-terminal aromatic motif (LFFGF, gray surface mode), stabilizes interaction of the nodulin domain with membranes. Charge neutralization of Lys residues by PtdIns(4,5)P2 promotes assembly of AtSfh1 into higher-order oligomers by interaction of the helical coiled-coil motifs (red ribbon diagram). (B) Engineering phosphoinositide signaling with point resolution. Distinct classes of Sec14-nodulins (Sec14 domains, rectangles; nodulin domains, ovals) scaffold PtdIns and PtdIns-phosphate kinase assemblies (gold and purple triangles, respectively) with distinct phosphoinositide effectors (bolts). Classes of individual complexes that prosecute distinct biological outcomes for phosphoinositide signaling are organized into signaling pixels denoted by open circles of different color. Phosphoinositide phosphatases (blue PacMan) hydrolyze phosphoinositides that escape pixel boundaries.