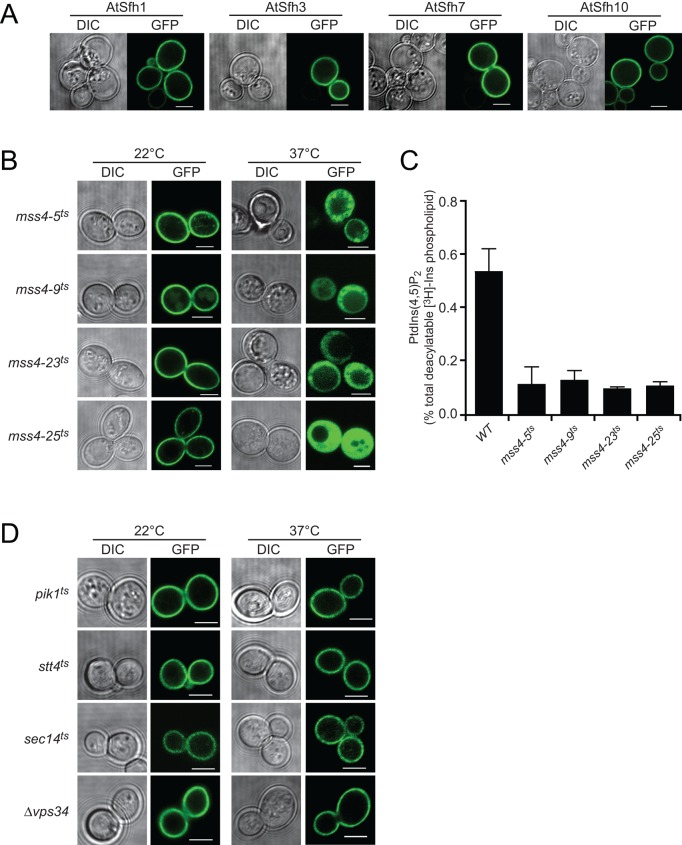
FIGURE 2:
Class I nodulins localize to yeast PM in a PtdIns(4,5)P2-dependent manner. (A) GFP-tagged class I chimeras localize to PM when expressed in WT yeast. Images are representative of 158, 261, 352, and 216 cells expressing AtSfh1, AtSfh3, AtSfh7, and AtSfh10 nodulins, respectively. All cells showed exclusive PM localization. (B) GFP-tagged class I nodulins are released from PM in four independently isolated mss4ts mutants when Mss4 is inactivated at 37°C. Images are representative of an aggregate of 901 and 1094 cells imaged at 22 and 37°C, respectively, and 107–355 cells were scored for each nodulin at each temperature. In all cases, >95% of the cells imaged at 22°C showed PM localization of the GFP-nodulin reporter, whereas, in all cases, >93% of the cells imaged at 37°C showed exclusively cytoplasmic localization for the indicated GFP-nodulin reporter. GFP-nodulin profiles were also imaged in WT yeast at 22°C (aggregate of 275 cells) and 37°C (aggregate of 184 cells). As expected, at both temperatures, 100% of the cells showed exclusively PM localization profiles. (C) Quantification of PtdIns(4,5)P2 in WT and mss4ts strains at 37°C. Analyses involved steady-state radiolabeling of cells with [3H]inositol at 22°C and shift of cells to 37°C for 2 h; total deacylated 3H-labeled inositol glycerolipids were quantified by anion-exchange HPLC. PtdIns(4,5)P2 values are expressed as percentage of total deacylatable [3H]inositol lipid. The unpaired t test p value (mutant compared with WT) is <0.012. (D) GFP-tagged class I nodulins localize to PM when expressed in yeast with temperature sensitive PtdIns-4-OH kinase (stt4ts and pik1ts), PtdIns-3-OH kinase (vps34ts), and Sec14 (sec14ts) incubated at 22 and 37°C. Differential interference contrast (DIC) and GFP confocal images are identified, and 107–170 cells were imaged for each mutant at each temperature. For each mutant and condition, >91% of the cells imaged showed exclusive PM localization for the indicated GFP-nodulin reporter. Scale bars, 2 μm.