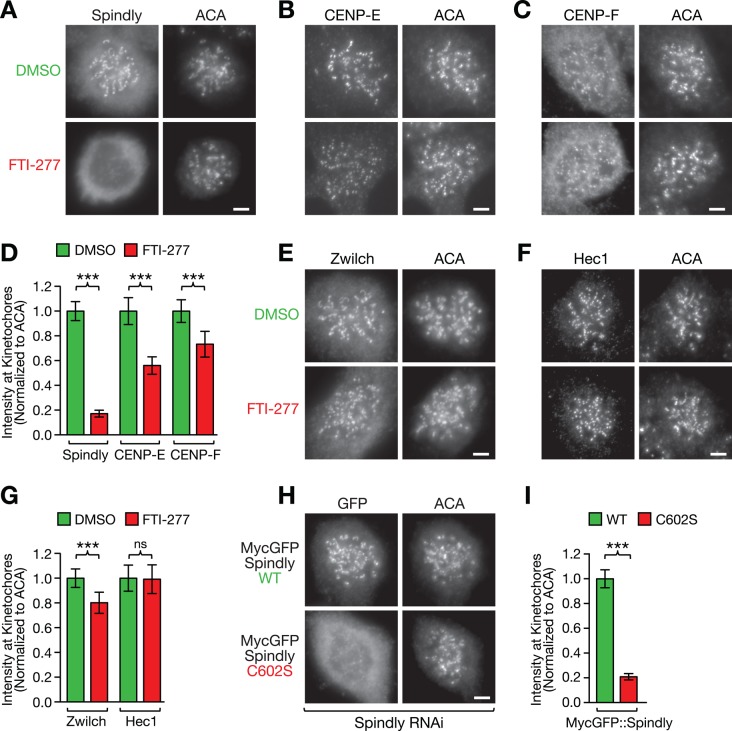
FIGURE 2:
Farnesylation of Spindly is required for its localization to microtubule-unattached kinetochores. (A–C) HeLa cells immunostained for the kinetochore proteins Spindly (A), CENP-E (B), and CENP-F (C) after treatment for 48 h with 10 μM farnesyltransferase inhibitor FTI-277 or DMSO. Cells were incubated in 1 μM nocodazole for 4 h to maximize the accumulation of the proteins at kinetochores and costained with anti-centromere antibodies (ACAs). Scale bars, 5 μm. (D) Quantification of protein levels at kinetochores in the conditions shown in A–C using immunofluorescence intensity measurements. Each condition represents a total of 100 kinetochore measurements from 20 different cells. Error bars represent the SEM with a 95% confidence interval. The t test was used to determine statistical significance (***p < 0.0001). (E, F) HeLa cells immunostained for the kinetochore proteins Zwilch (E) and Hec1 (F) after treatment for 48 h with 10 μM farnesyltransferase inhibitor FTI-277 or DMSO. Scale bars, 5 μm. (G) Kinetochore level quantification of the conditions in E and F displayed as described for D (ns, not statistically significant). (H) Kinetochore localization of RNAi-resistant, MycGFP-tagged wild-type (WT) and mutant (C602S) Spindly in nocodazole-treated HeLa cells after depletion of endogenous Spindly, visualized by immunofluorescence with an anti-GFP antibody (see Figure 5A for corresponding RNAi immunoblot). Scale bar, 5 μm. (I) Kinetochore level quantification of the condition in H displayed as described for D.