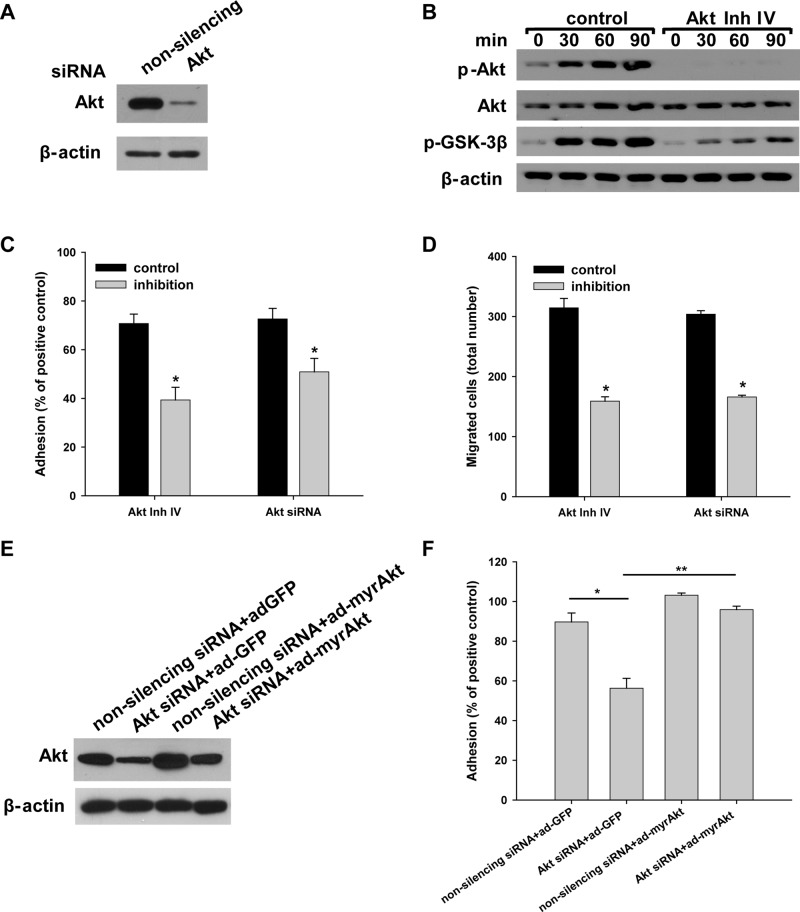
FIGURE 5:
Integrin α3β1–dependent adhesion and migration on LM-332 are regulated by Akt activation. Itgα3f/f CD cells were transfected with nonsilencing or Akt siRNA (100 nM for 48 h) (A and C–F) or treated with the Akt inhibitor IV (5 μM for 1 h) or DMSO (control) (B–D). The effect of the siRNA on AKT expression is shown by immunoblotting cell lysates for total Akt, with β-actin serving as a loading control (A). (B–D) Control cells or cells treated with Akt inhibitor IV were subjected to replating, after which cell lysates were immunoblotted for phosphorylated AktSer473 and GSK-3β and total Akt (20 μg total protein/lane), with β-actin serving as a loading control (B). Treated and untreated cells were evaluated for adhesion (C) or migration (D) on LM-332 (1 μg/ml) and evaluated as described in Materials and Methods. Mean measurements ± SEM of four to six independent experiments are shown; *, p ≤ 0.05 between control Itgα3f/f cells and Itgα3f/f cells with inhibitors. (E and F) Itgα3f/f CD cells were transfected with either nonsilencing or Akt siRNA, with control (ad-GFP) or myristilated Akt (100 plaque-forming units/cell for 24 h) (myrAkt) (E) and subjected to an adhesion assay as described in C (F). Mean measurements ± SEM of four to six independent experiments are shown; *, p ≤ 0.05 between control Itgα3f/f cells and Itgα3f/f cells transfected with Akt siRNA; *, p ≤ 0.05 between Itgα3f/f cells with Akt siRNA and Itgα3f/f cells with Akt siRNA and myristilated Akt.