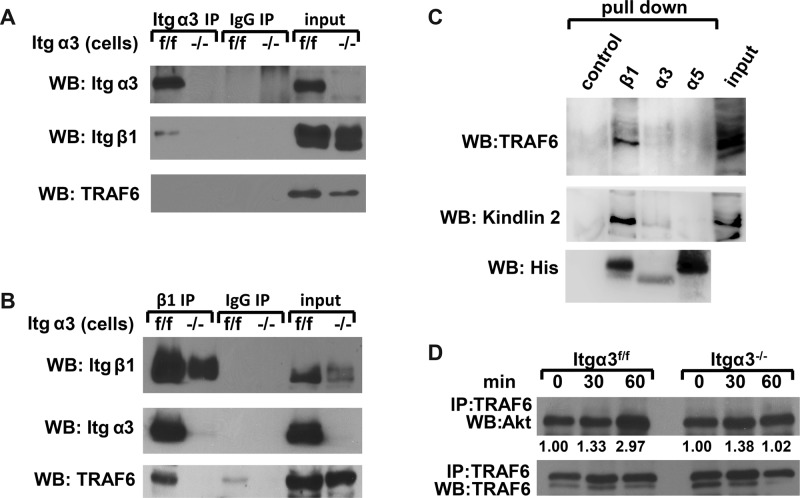
FIGURE 9:
TRAF6 forms a complex with α3β1 integrin and Akt. (A and B) Cell lysates from Itgα3f/f and Itgα3−/− CD cells (1.0 mg total protein) were immunoprecipitated with protein G-Sepharose–coupled antibody to α3 (A) or β1 (B) integrin subunits. Immunoprecipitates were subjected to Western blot analysis with antibodies to TRAF6 or α3 or β1 integrin subunits. Input was 20 μg total protein lysates (2%). (C) Ni-NTA magnetic agarose beads (control), α3-TM-Cyto domains, α5-TM-Cyto domains, or β1-TM-Cyto domains bound to Ni-NTA magnetic agarose beads were incubated with Itgα3f/f cell lysates and then immunoblotted with antibodies to TRAF6, kindlin 2, or His. (D) Itgα3f/f and Itgα3−/− CD cells were trypsinized and replated on LM-332 (1 μg/ml) as described in Figure 4A. Cell lysates (200 μg total protein) were immunoprecipitated with antibodies to TRAF6 and immunoblotted for Akt or TRAF6. Levels of Akt and TRAF6 were measured by densitometry, normalized to TRAF6 levels, and expressed as fold change relative to cells left in suspension (0 time point). Values shown are representative of three experiments.