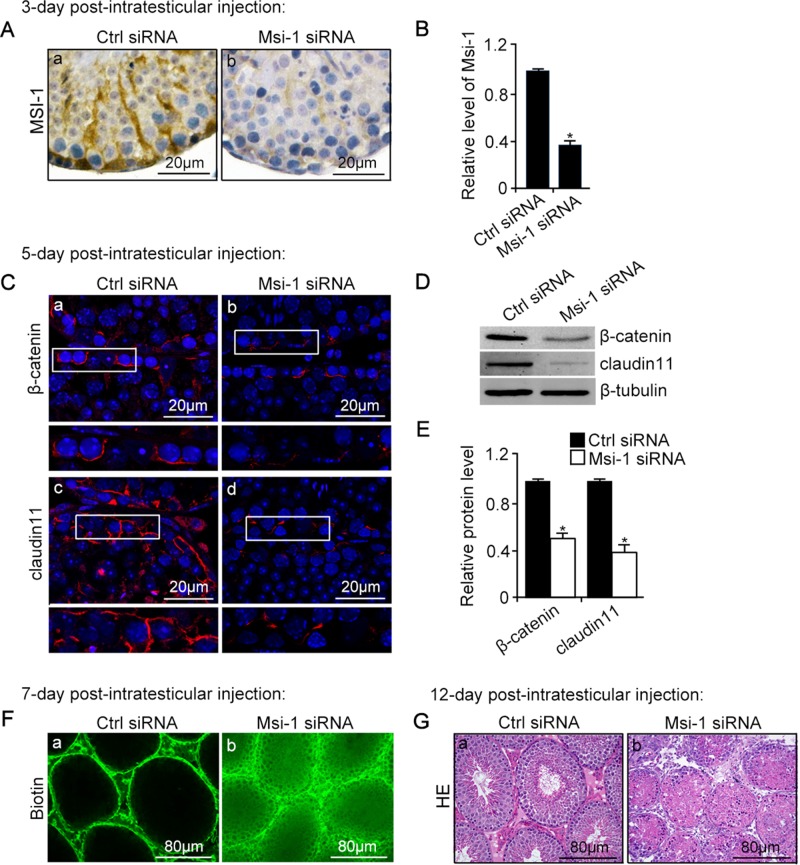
FIGURE 3:
An in vivo study assessing the role of Msi-1 in BTB function and spermatogenesis by RNAi intratesticular injection. Msi-1–targeting siRNA duplexes and nontargeting duplexes were administered to each testis in adult mice as a single treatment. The knockdown efficiency, BTB-associated protein expression, biotin tracer experiment, and hematoxylin-eosin (HE) staining at 3, 5, 9, and 12 d postintratesticular injection, respectively. (A) Considerably more intense Msi-1 staining was observed in the Sertoli cells from testes transfected with nontargeting control (a) versus testes transfected with Msi-1–specific siRNA duplexes (b). Scale bars: 20 μm. (B) Histogram summarizing immunoblotting results of Msi-1 and normalized against β-tubulin. Each bar represents the mean ± SD of n = 3 experiments. *, p < 0.05. (C) Paraffin sections were used to study changes in the expression of β-catenin (a and b) and claudin-11 (c and d) after Msi-1 knockdown in vivo. The boxed area was randomly selected. Scale bars: 20 μm. (D) The protein levels of β-catenin and claudin-11 in nontargeting control and Msi-1 knockdown testes. β-tubulin served as a loading control. (E) Histogram summarizing selected immunoblotting results in D and normalized against β-tubulin. Each bar represents the mean ± SD of n = 3 experiments. *, p < 0.05. (F) We injected a biotin tracer into the testes of live anesthetized mice (n = 3) and examined the subsequent changes in BTB integrity after siRNA duplexes injection. (G) HE staining showed that the continuous process of spermatogenesis was seriously disrupted after Msi-1 knockdown in vivo. Scale bars: 80 μm.