Abstract
Peroral endoscopic myotomy (POEM) incorporates concepts of natural orifice translumenal endoscopic surgery and achieves endoscopic myotomy by utilizing a submucosal tunnel as an operating space. Although intended for the palliation of symptoms of achalasia, there is mounting data to suggest it is also efficacious in the management of spastic esophageal disorders. The technique requires an understanding of the pathophysiology of esophageal motility disorders as well as knowledge of surgical anatomy of the foregut. POEM achieves short term response in 82% to 100% of patients with minimal risk of adverse events. In addition, it appears to be effective and safe even at the extremes of age and regardless of prior therapy undertaken. Although infrequent, the ability of the endoscopist to manage an intraprocedural adverse event is critical as failure to do so could result in significant morbidity. The major late adverse event is gastroesophageal reflux which appears to occur in 20% to 46% of patients. Research is being conducted to clarify the optimal technique for POEM and a personalized approach by measuring intraprocedural esophagogastric junction distensibility appears promising. In addition to esophageal disorders, POEM is being studied in the management of gastroparesis (gastric pyloromyotomy) with initial reports demonstrating technical feasibility. Although POEM represents a paradigm shift the management of esophageal motility disorders, the results of prospective randomized controlled trials with long-term follow up are eagerly awaited.
Keywords: Peroral endoscopic myotomy, Achalasia, Myotomy, Dysphagia
Core tip: Peroral endoscopic myotomy (POEM) is a minimally invasive, scarless approach to Heller myotomy for the palliation of symptoms of achalasia and spastic esophageal disorders. Current data demonstrates short-term success with minimal adverse events. POEM is no longer considered experimental with approximately 5000 procedures performed worldwide. In the future, a personalized approach to POEM will be undertaken with tailoring of the length of gastric myotomy based on intraprocedural physiological measurements. This will allow sufficient reduction in pressure at the lower esophageal sphincter for adequate relief of symptoms but also minimize gastroesophageal reflux.
INTRODUCTION
Peroral endoscopic myotomy (POEM) is a minimally invasive endoscopic procedure intended for long-term recovery from symptoms of esophageal achalasia. Achalasia is a benign motility disorder of the esophagus which is characterized by incomplete relaxation of the lower esophageal sphincter (LES) and aperistalsis of the esophageal body. As the etiology of achalasia is not known, all treatment options are directed at decreasing the resting pressure at the LES.
The first reported endoscopic myotomy in humans was published in 1980[1]. In this report, the myotomy was carried out in an uncontrolled manner by incising the mucosa through to deeper layers of the lower esophageal sphincter with a needle knife. The method achieved technical success in all 17 patients although there was concern as the wound was directly exposed to esophageal and gastric contents and if too deep, there would be a direct perforation to the mediastinum and/or peritoneum. There were three minor bleeding episodes which were controlled endoscopically. The clinical, manometric and radiological postoperative results were promising. However, the direct incision method was not considered a reliable and safe approach and was therefore not adopted by the medical community.
Developments in natural orifice translumenal endoscopic surgery (NOTES)[2] have resulted in a submucosal endolumenal approach for the treatment of achalasia using POEM. Sumiyama et al[3] was the first to describe the idea of submucosal tunneling. However, Pasricha et al[4] initially described the concept of POEM in 2007 in an experimental preclinical model. This report demonstrated the safety and efficacy of performing a myotomy under endoscopic visualization in 4 pigs after the formation of a submucosal tunnel. Inoue et al[5] championed translating this innovative technique into clinical care in 2010 with the first human study reporting favorable results in 17 achalasia patients.
Multiple studies from Asia, Europe and the United States reveal that POEM is a safe and effective therapy for achalasia when performed by expert endoscopists. In addition, the recent white paper summary from the American Society of Gastrointestinal Endoscopy provided substantial data to support the notion that POEM is a promising therapeutic modality[6]. This review illustrates the patient selection and preparation, operative technique, clinical outcomes and future directions for POEM.
PATIENT SELECTION AND INDICATIONS
All patients with symptomatic, manometrically proven, primary idiopathic achalasia are eligible candidates to undergo POEM. Among initial published clinical studies, exclusion criteria included previous esophageal or gastric surgery (including Heller myotomy), sigmoid type esophagus, age under 18 years or inability to undergo general anesthesia. Other less common scenarios that rendered patients unsuitable for POEM included severe erosive esophagitis, significant coagulation disorders, liver cirrhosis with portal hypertension or prior therapy that may compromise the integrity of the esophageal mucosa or could have led to submucosal fibrosis (radiation, endoscopic mucosal resection, radiofrequency ablation, etc.). Previous therapy, such as uncomplicated pneumatic balloon dilation and botulinum toxin injection are not contraindications to POEM, although, in these cases inflammatory fibrosis is often encountered during submucosal dissection.
There are now multiple series reporting the technical success, efficacy and safety of POEM in patients who have undergone a prior Heller myotomy[7-9]. Successful POEM in the setting of a Roux-en-Y gastric bypass has also been reported[10] where the extensive adhesions and altered anatomy could have proven challenging for the surgical approach. POEM has also been studied in patients with with sigmoid-type achalasia with similar outcomes as those with a non-sigmoid type esophagus[11]. Age is no longer a contraindication to POEM with successful procedures being performed in those even at the extremes of age. In particular, several series have reported its successful use in the pediatric population[12,13].
Though POEM is classically done for the palliation of symptoms of achalasia, it is being increasingly used for the treatment of other foregut disorders. There are growing reports supporting its use in spastic esophageal disorders such as diffuse esophageal spasm (DES) and Jackhammer esophagus[14-19]. It is potentially more efficacious than even surgical myotomy as it allows myotomy not only of the LES, but also of the esophageal body, where hypertensive contractions occur[15,20,21]. Additionally, POEM has even been reported in the stomach (endoscopic pyloromyotomy) as a treatment strategy for selected patients with gastroparesis[22,23].
EVALUATION AND PREPARATION
It is obligatory that patients have a diagnosis of achalasia or spastic esophageal disorder firmly established based on clinical history, esophageal manometry, contrast esophagram and esophagogastroduodenoscopy (EGD). A standardized validated symptom assessment form is completed by all patients, with the majority of centers using the Eckardt score[24]. The Chicago classification of esophageal motility disorders mandates high resolution esophageal manometry (HREM) to identify the precise achalasia or spastic esophageal disorder subtype, although the clinical significance of this classification has been recently debated[25]. Computed tomography (CT) is not mandatory, however, some experts find it useful as it provides information on the anatomic features of adjacent structures as well as identifying congenital anomalies or ectopic varices. In addition, CT scan can be an adjunct to contrast esophagram in establishing the presence of a sigmoid-type esophagus.
Our institutional protocol is to perform an EGD on all patients 2 wk prior to their procedure. All patients are placed on a clear liquid diet 2 d prior to this endoscopy and an endoscopic assessment is made of the quantity of residual esophageal contents. If there is persistent solid residue, then even more stringent dietary restriction is advised prior to POEM. This will allow for a clear endoscopic view and may avoid aspiration during induction of anesthesia. Additionally, this evaluation allows for exclusion of underlying malignancy, erosive esophagitis, Barrett’s esophagus and Candidal esophagitis. On occasion, a HREM catheter is inserted as passage without endoscopic guidance can be challenging in patients with a tight LES and/or sigmoid esophagus.
In case of use of anticoagulant or antiplatelet therapy, it is generally recommended that these medications be stopped with the exception of acetylsalicylic acid when prescribed as secondary prophylaxis. All patients have a blood type and screen performed on the day of the procedure.
POEM PROCEDURE
Our institutional protocol has been to perform POEM in the endoscopy suite. This is in contrast to most other centers where POEM is performed in the operating room[11,26]. We have performed over 50 cases in the endoscopy unit without a major intraprocedural adverse event. The procedure is performed with the patient in the supine position under general anesthesia with endotracheal intubation and complete paralysis. Our protocol is to use a specialized endotracheal tube that has a taper-shaped cuff with an evacuation port and suction lumen (TaperGuard Evac, Covidien, Mansfield, MA, United States). We have noted that approximately 100mls of fluid is aspirated through this specialized endotracheal tube during each procedure with no episodes of aspiration or pneumonia in our cohort[27].
Carbon dioxide (CO2) insufflation is mandatory for safe POEM and to reduce the risk of mediastinal emphysema, tension pneumoperitoneum, pneumothorax and air embolization. An arterial line may be inserted such that an arterial blood gas can be performed and CO2 levels monitored as needed. An indwelling urinary catheter is inserted and a forced air warming blanket is used to cover from the waist down.
The patient’s abdomen remains in unrestricted view to allow for an immediate diagnosis of severe pneumoperitoneum. The abdomen is palpated periodically and if tidal volumes begin to diminish or the abdomen is markedly distended, decompression is accomplished using a Veress needle.
A thorough cleansing of the esophageal lumen is performed prior to commencement of the intervention. In certain cases, a therapeutic gastroscope with a 3.8mm working channel (GIF-Q260J; Olympus, Center Valley, PA, United States) equipped with a water jet is necessary. If adherent residue is present on the esophageal mucosa, a soft cleaning cap (Barrx RFA Cleaning Cap - Medium: CP-002A, BARRX Medical Inc., Sunnyvale, CA) can be used to safely scrape off the residue. Broad-spectrum intravenous antibiotics are administered.
Measurements of esophagogastric junction distensibility can be performed using the endoluminal functional lumen-imaging probe (EndoFLIP; Crospon, Galway, Ireland) (Figure 1). This provides a baseline by which the operator can assess the adequacy of the myotomy and may play a role in predicting which patients will likely be non-responders[28-30]. Subsequently, the esophagus is lavaged with 240 mL of sterile saline solution mixed with 180 mg of gentamicin.
Figure 1.
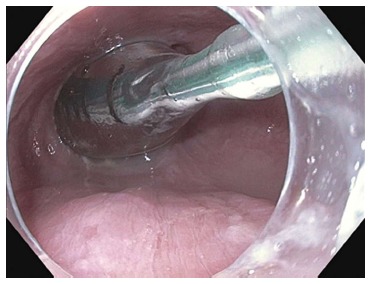
Endosocopic image of the endoluminal functional lumen imaging probe assessing the esophagogastric junction via impedence planimetry.
A high-definition gastroscope fitted with a transparent cap is used. It is recommend to secure the cap on the endoscope tip with tape as anecdotal reports exist of dislodgement of the cap within the submucosal tunnel. A gastroscope that has a dedicated water jet channel such as the GIF-HQ190/GIF-H180J (Olympus, Center Valley, PA, United States) or EG2990i/EG2990k (Pentax Medical Corp., Montvale, New Jersey, United States) is recommended. For all our procedures, a bottle filled with saline and a second bottle of saline mixed with indigo carmine are attached to the water jet channel via a stopcock. Individual foot pedals activate each bottle[31] (Figure 2).
Figure 2.
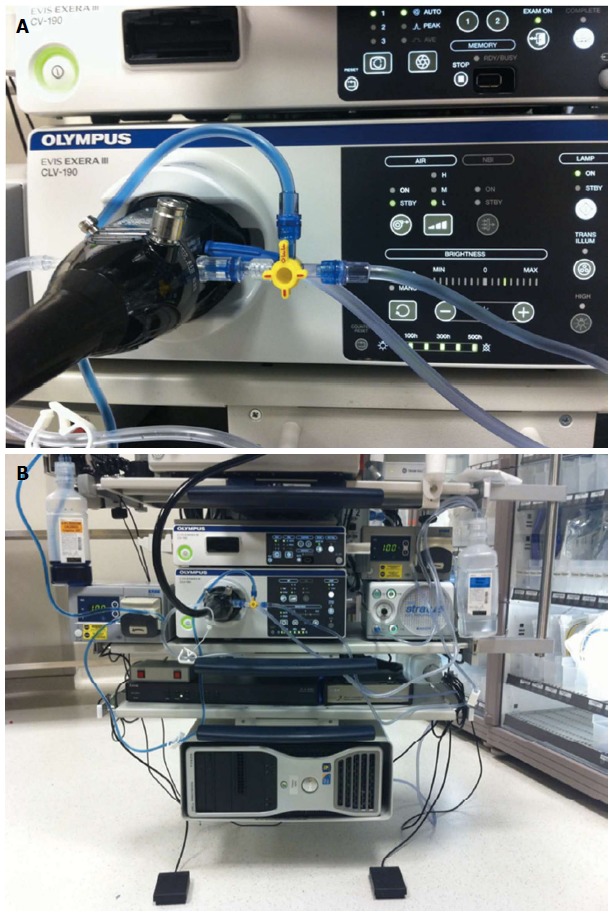
Endoscope setup for jet injection of dyed saline. A: One bottle of saline and a second bottle of saline mixed with indigo carmine are directly connected to the water jet channel via a stopcock; B: Separate foot pedals control injection of either pure saline for optimizing visual field or dyed saline for submucosal tunneling.
Four step approach to poem
The procedure can be split into four consecutive steps: the mucosal incision, formation of the submucosal tunnel, myotomy and closure of the mucosal incision[32].
Step 1: Mucosal incision: The level of the esophagogastric junction (EGJ) is identified and determines the level submucosal tunneling is commenced. In most centers, an anterior (2 o’clock position) is used for the submucosal tunnel and myotomy. However, in some centers a posterior orientation (5 o’clock position) is favored. An anterior myotomy may decrease the damage to the angle of His, a barrier to post-operative GERD. If there is doubt as to the identification of the anterior and posterior walls, water can be injected into the esophageal lumen and will pool on the posterior aspect when the patient is positioned supine.
A submucosal cushion is then made 3 cm proximal to the proposed commencement of myotomy using 0.01% epinephrine, 0.25% indigo carmine and 0.9% saline solution. A 1.5 cm vertical mucosal incision is made using a hybridKnife (HK) (ERBE, Tubingen, Germany) or triangular tip (TT) knife (KD 640 L, Olympus, Center Valley, PA, United States) using a dry cut mode at 50 W on effect 3 (ERBE, Tubingen, Germany) (Figure 3). The gastroscope is then inserted into the submucosal space after dissection of the submucosal fibers at the level of the mucosal incision.
Figure 3.
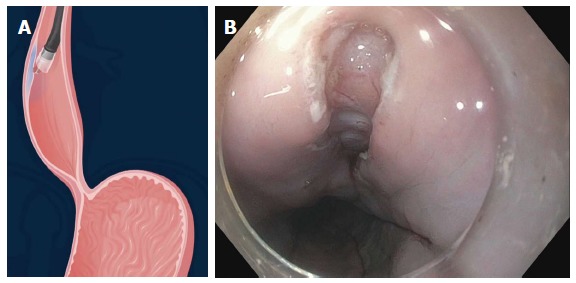
Longitudinal mucosal incision 1.5 to 2 cm on the anterior esophageal wall.
The length of the submucosal tunnel (and hence myotomy) must be determined prior to commencement of the mucosal incision. In patients with achalasia subtype I and II, a 6-10 cm esophageal myotomy is performed. In patients with spastic esophageal disorders, the length of myotomy is determined based on the proximal extent of the hypertensive contractions on HREM and/or the level of visible spastic contractions seen endoscopically.
Step 2: Creation of submucosal tunnel: The submucosal tunnel is created distally using a technique similar to endoscopic submucosal dissection. Using a TT knife or HK, the submucosa is dissected with a no-touch technique using spray coagulation mode at 50 W on effect 2 (ERBE, Tubingen, Germany) (Figure 4). The dissection plane is located nearly on the surface of the muscularis propria. Recurrent jet injection of indigo carmine mixed with saline is done to increase the delineation between the submucosal fibers and muscularis propria whenever the planes become ambiguous (Figure 5). Care must be taken to avoid injury to the mucosal layer during creation of the submucosal tunnel as the mucosal layer is the only barrier between the esophageal lumen and mediastinum after myotomy. Large vessels in the submucosa are coagulated using the Coagrasper (Olympus, Center Valley, PA, United States) in soft coagulation mode at 80 W on effect 5 (ERBE, Tubingen, Germany).
Figure 4.
Creation of the submucosal tunnel. A: Dissection of the submucosal fibers using spray coagulation with the triangular tip knife (KD 640L, Olympus, Center Valley, PA, United States); B: Dissection of the submucosal fibers using the hybridKnife (ERBE, Tubingen, Germany) which allows for both submucosal dissection and fluid injection into the submucosal space.
Figure 5.
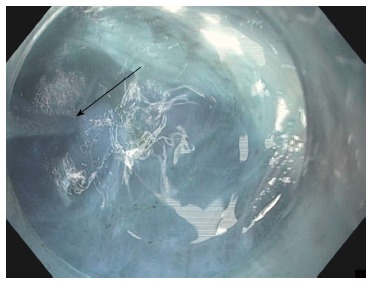
Repeated jet injection (arrow) of dyed saline is performed during submucosal tunneling to improve the demarcation between the submucosal layer and muscularis propria whenever the submucosal dissection plane becomes unclear.
An alternative technique for centers that do not have a water jet for injection is to use the HK (ERBE, Tubingen, Germany). This device obviates the need for multiple accessory exchanges between needle injector and knife as needless submucosal injection using a high-pressure water jet and electrosurgical dissection can both be performed. A randomized controlled trial demonstrated that the HK resulted in statistically significant quicker operating times as compared to using the TT knife. This was primarily the result of a statistically significant lower average number of accessory exchanges during the procedure (2 vs 19.2, P < 0.0001)[33]. However, the above-described water jet injection method obviates the need for these frequent exchanges as well.
Another technique uses a balloon, such as a standard biliary stone extraction balloon, to dissect the submucosal fibers without the use of electrosurgery. Operators who use this technique claim that it allows for a more rapid creation of the tunnel without substantial bleeding as the vessels are momentarily displaced rather than ruptured using this technique. Proponents of this technique also state that this method is particularly useful at the LES when the space between the muscle and mucosal layer is limited.
The submucosal tunnel is extended 3cm beyond the EGJ (Figure 6). This is essential as an adequate gastric myotomy is considered critical to eradicate the sling and clasp fibers which are considered essential to maintain LES continence[34,35]. The techniques to assess the location of the EGJ include: insertion depth, narrowing of the submucosal space and resistance of passage of the endoscope through the EGJ followed by prompt expansion of the space at the gastric cardia, change in vasculature, visualization of aberrant longitudinal muscle fibers at the EGJ and injection of epinephrine or indocyanine green (ICG)[36,37]. Many of these methods are subjective. Our preference is to use one of two objective techniques: double endoscope transillumination technique or intraprocedural fluoroscopy[38,39].
Figure 6.
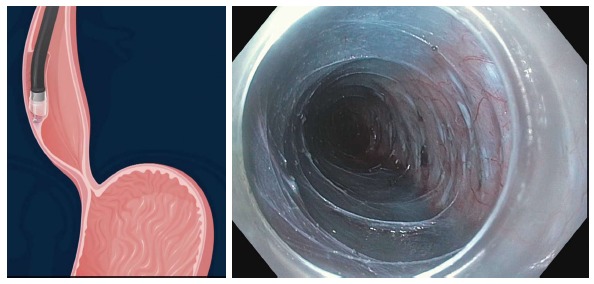
Submucosal tunnel after the submucosal fibers have been dissected away. The myotomy can now be commenced.
Double endoscope transillumination for extent confirmation technique
After the submucosal tunnel is created, the gastroscope is withdrawn and an ultraslim, 5.9-mm endoscope is inserted through the mouth into the stomach and then retroflexed. The cap fitted gastroscope is reinserted alongside the ultraslim gastroscope into the submucosal tunnel. The brightness of the light of the ultraslim gastroscope is reduced while transillumination is switched on for the standard gastroscope within the submucosal tunnel. The transilluminated light is seen by the ultraslim gastroscope and hence allows exact appreciation of the extent of the tunnel into the proximal stomach (Figure 7).
Figure 7.
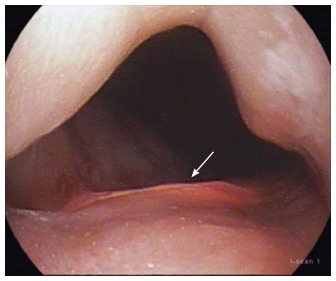
Transillumination can be seen 2 cm below the gastroesophageal junction indicating that the submucosal tunnel can be extended a further 1 cm prior to commencing the myotomy.
Intraprocedural fluoroscopy
After the formation of a submucosal tunnel, a radio-opaque marker (endoscopic clip placed at EGJ on the opposite side to the submucosal tunnel or fluoroscopically guided placement of a 19-gauge needle on the skin) is used to mark the EGJ. The endoscope is then re-inserted to the terminal aspect of the submucosal tunnel. Using a C-arm, a fluoroscopic image is obtained in the anterior-posterior axis. The distance between the jaws of the endoscopic clip or needle, and the endoscope tip is calculated using the known diameter of the endoscope as a scale (Figure 8). This allows for an objective measurement of the length of the submucosal tunnel below the EGJ.
Figure 8.
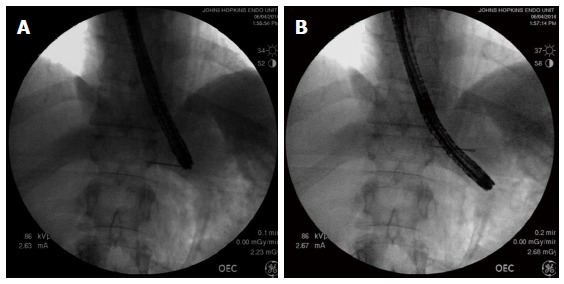
Using fluoroscopy to assess the adequacy of gastric myotomy during peroral endoscopic myotomy. A: The needle is fluoroscopically lined up with the tip of the endoscope and leveled with the EGJ; B: The endoscope has a diameter of 1 cm, and therefore, the endoscope tip is measured to be 3 cm below the needle marking the EGJ. EGJ: Esophagogastric junction.
Step 3: Myotomy: Selective myotomy of the inner circular muscle is performed 1cm below the end of the mucosal incision. The HK or TT knife is used to grasp and lift circular muscle fibers followed by cutting with spray coagulation current at 50 W on effect 2 (ERBE, Tubingen, Germany). Selective myotomy of the inner circular muscle, preserving the outer longitudinal esophageal muscular layer is usually preferred during POEM (Figure 9). The selective circular muscle myotomy is intended to prevent the endoscope entering the pleural space and decrease morbidity. However, it can be difficult to accomplish because the longitudinal muscle fibers of the esophagus are very thin, and therefore inadvertent splitting of these fibers often occur during POEM. Either trauma from maneuvering the endoscope in the tunnel, electrocautery damage, or CO2 insufflation alone can result in splitting of the longitudinal muscle layer and adventitia and hence direct exposure to the mediastinum or peritoneum[32] (Figure 10). Moreover, the ability to differentiate between circular and longitudinal muscular layers becomes particularly challenging at the level of the EGJ and stomach. Therefore, some experts are of the opinion that a full-thickness myotomy at this level is mandated for adequate and long-term reduction of pressure at the LES.
Figure 9.
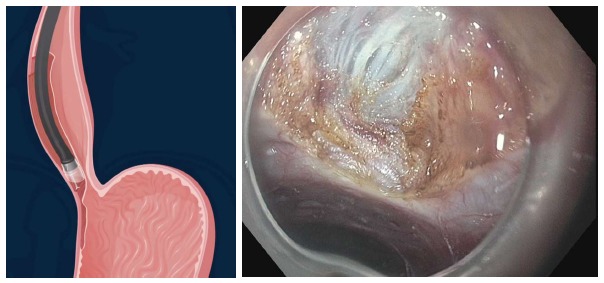
Selective myotomy of the circular muscle fibers. The longitudinal muscle fibers have been preserved.
Figure 10.
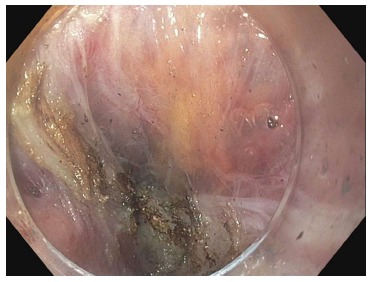
Splitting of the longitudinal muscle fibers despite attempted selective cardiomyotomy. Peritoneal fat can be seen through the translucent adventitia.
Li et al[40] compared the outcomes between 131 patients that underwent selective inner circular muscle myotomy and 103 who underwent full-thickness myotomy. The average procedure times were briefer in the full-thickness myotomy cohort (42 min vs 49 min, P = 0.02). No difference was found in the frequency of adverse events between the cohorts. During follow-up, clinical success (Eckardt score ≤ 3) persisted for 115/121 (95.0%) of patients in selective inner circular myotomy cohort and 95/99 (96.0%) of patients in full-thickness myotomy cohort (P = 0.75). There were no significant differences in absolute (pre and post) or mean reduction in LES pressures between groups (both P > 0.05). There was no statistically significant difference in the rate of clinical reflux events (21.2% vs 16.5%, P = 0.38). The authors concluded that there was no meaningful difference between the two methods in terms of symptom relief and manometric outcomes.
It is believed that selective inner circular myotomy adds an element of extra safety to POEM and hence may lead to easier dissemination, especially when performed by endoscopists with lesser experience. Although the myotomy is not the most challenging part of the procedure, it must be performed carefully such that there is adequate separation of muscle fibers and inadvertent damage to vessels is avoided. Once the myotomy is completed, smooth passage of the endoscope through the area of the LES into the stomach should provide confirmation of complete myotomy.
Step 4: Closure of the mucosal incision: Prior to closure of the mucosal incision, a careful inspection of the submucosal tunnel is performed and any oozing is controlled. Then the esophageal mucosa is interrogated and any laceration or mucosotomy is addressed. Lower esophageal sphincter relaxation is evaluated by retroflexed visualization of the gastric cardia. Repeat EndoFLIP measurements can now be performed to determine post myotomy distensibility.
The mucosal entry can be closed with endoscopic clips[37,41] or the use of a flexible endoscopic suturing device (OverStitch; Apollo Endosurgery, Austin, TX, United States)[42] (Figure 11). When endoscopic clips are used, the initial clip is deployed at the most distal part of the mucosal incision to facilitate approximation of the incisional borders. Placement of subsequent clips is performed in a proximal direction until complete closure. Salvage closure techniques have been reported when standard methods fail. These include over-the-scope clip and a covered esophageal stent[43-45].
Figure 11.
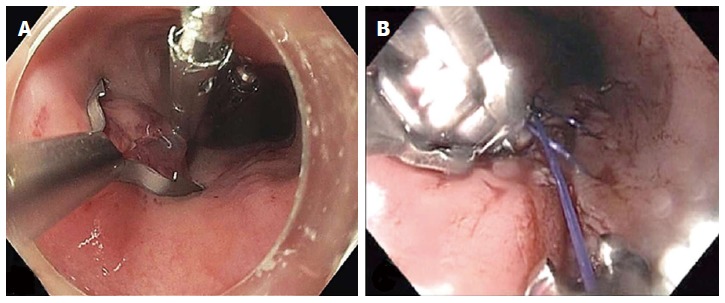
Closure of the mucosal entry after completion of the myotomy. A: A posterior mucosal incision was closed with three endoscopic clips; B: A posterior mucosal incision was closed with a running suture pattern using transoral flexible endoscopic suturing (OverStitch; Apollo Endosurgery, Austin, TX, United States).
POSTOPERATIVE CARE
Patients are admitted overnight for observation and kept nil per oral. Intravenous prophylactic antiemetics and broad-spectrum antibiotics are prescribed. A contrast esophagram is obtained the following morning and a soft diet is commenced after exclusion of an esophageal leak. Routine thoracic CT scan is not warranted because of the high rate of minor and clinically irrelevant findings[46]. Patients are routinely prescribed broad-spectrum antibiotics for 5 to 7 d. Patients remain on soft diet for 2 wk after which a normal diet can be commenced. In order to avoid any potential damage to the esophageal mucosa, we prescribe twice daily proton pump inhibitor for 2 wk. Follow-up clinic visit to assess for delayed complications and assessment of clinical response (Eckardt score) occur at 3 mo post procedure. Additionally, repeat HREM and esophageal acid exposure testing are ordered routinely.
EFFICACY OF POEM
The published literature to date illustrates that POEM is highly effective in the short-term management of achalasia. In the almost all series, clinical success was defined as postprocedure Eckardt score ≤ 3. It should be noted that a patient could suffer dysphagia at each meal despite an Eckardt score of 3. Other metrics used are decrease in LES pressure, improvement in esophageal emptying and quality of life. It must be noted that most data are derived from studies that are uncontrolled and open label. Additionally, the follow-up interval is often short and frequently not standardized. Only one prospective multicenter study exists which reports outcomes to 12 mo[41]. The recent white paper summarized data from 14 studies with outcomes based on 804 patients[6]. Clinical success was reported in 82% to 100% of patients with significant reductions in Eckardt score and LES pressure. Several studies have described efficacy based on timed barium esophagram (an objective measurement of esophageal emptying)[42,47,48]. Recent reports have also documented a significant improvement in several measures of quality of life after POEM[42,49].
EndoFLIP has been increasingly reported as a method of assessing the adequacy of myotomy during the POEM procedure. This method involves using a balloon catheter outfitted with a series of electrodes that is placed across the EGJ and allows measurement of luminal diameter, cross-sectional area and balloon pressure via impedence planimetry (Figure 12). An index of EGJ distensibility can be determined and this has been shown to correlate better with postoperative symptoms than manometric pressure measurements[50]. Patients within a “sweet spot” of postoperative EGJ distensibility (4.5-8.5 mm2/mmHg) are almost twice as likely to have optimal symptom outcomes as those outside this window[28].
Figure 12.
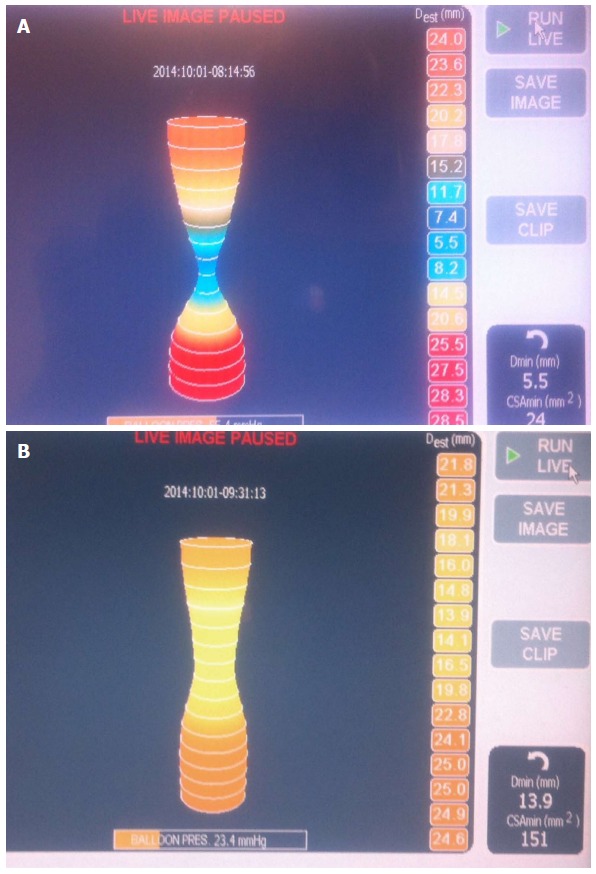
Endoluminal functional lumen-imaging probe (EndoFLIP; Crospon, Galway, Ireland). A: EndoFLIP measurements performed prior to commencement of POEM. A tight hourglass shape at the EGJ can be seen; B: On completion of the myotomy the waist is widened with a corresponding increase in the distensibility index. POEM: Peroral endoscopic myotomy; EGJ: Esophagogastric junction.
The literature to date supports the notion that POEM is feasible, safe and efficacious in patients that have undergone prior botulinum toxin injections or pneumatic balloon dilation and is comparable to patients who are treatment naive[51-53]. In the IPOEMS survey, 40% of patients had undergone POEM after prior endoscopic therapy[36]. The consensus from POEM operators is that botulinum toxin injections injections induce submucosal fibrosis and results in a more challenging dissection (which can be overcome by operator experience) with undiminished efficacy[36]. POEM after prior pneumatic balloon dilation did not render the procedure more technically challenging or increase the rate of complications and the efficacy was undiminished[36].
Relapse or persistence of symptoms after a Heller myotomy happens in 10% to 20% of patients at 2-year follow-up[54]. There are 3 studies that specifically examined the utility of POEM in patients that have undergone a prior Heller myotomy. A prospective study of 12 patients by Zhou et al[8] with relapse or persistence of symptoms after Heller myotomy underwent technically successful POEM after a mean of 12 years from the time of the primary Heller myotomy. No major adverse events related to POEM were encountered. Treatment success was achieved in 11/12 (91.7%) patients (mean Eckardt score pretreatment vs posttreatment: 9.2 vs 1.3; P < 0.001) at a mean follow-up of 10.4 mo. Onimaru et al[7] reported their series of 11 patients who had relapse or persistent achalasia and had undergone Heller myotomy as initial therapy. Ten patients underwent salvage POEM which was performed successfully without adverse events. One patient responded to pneumatic dilation. At 3 mo follow-up, a significant reduction in Eckardt symptom scores (6.5 vs 1.1, P < 0.001) and LES resting pressures (22.1 vs 10.9 mmHg, P < 0.01) were noted. Finally, Vigneswaran et al[9] reported 5 patients who had symptom recurrence after Heller myotomy and subsequently underwent POEM. The average procedure time was 149 min. In all patients, there was a significant reduction in average postprocedure Eckardt score (6.8 vs 0.6, P < 0.001). Therefore, its appears that POEM may be a worthwhile treatment option for patients with relapse or persistent symptoms after Heller myotomy.
While POEM is usually performed for the management of achalasia, preliminary data suggest it is a viable option for the management of spastic esophageal disorders since it permits myotomy of the proximal esophagus (where hypertensive contractions occur). It is has been suggested that in those patients, a greater improvement in dysphagia is noted compared to chest pain in patients undergoing POEM[21,55]. Based on expert opinion, it is recommended that a longer esophageal myotomy be performed for spastic esophageal disorders and the level of commencement should be based on the findings of HREM and endoscopy[15].
ADVERSE EVENTS
POEM is a safe endoscopic technique associated with a low rate of perioperative and postoperative adverse events when performed by experienced operators.
Intraprocedural adverse events
Subcutaneous emphysema and pneumoperitoneum are often encountered during the procedure and are no longer considered adverse events. Pneumothorax is infrequently encountered and does not usually require treatment as CO2 is rapidly absorbed. If there is respiratory compromise then a chest tube should be inserted and the procedure continued. In cases of tension pneumoperitoneum a Veress needle can be inserted through the abdominal wall. Excessive hypercarbia resulting from extended CO2 administration may require the endoscopist to momentarily remove the endoscope from the patient for 5 to 10 min.
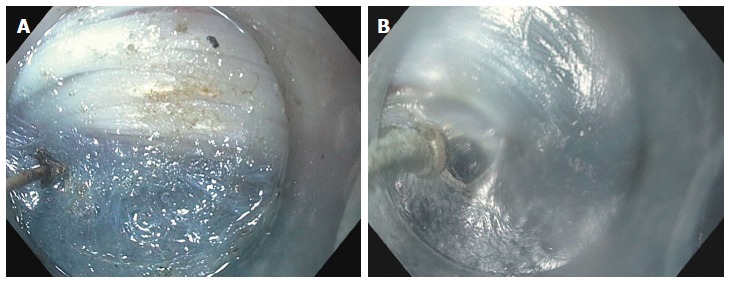
The most feared complication is an inadvertent mucosotomy which results in a perforation. As the submucosa and muscle have been dissected, even a small mucosotomy is potentially dangerous. Most mucostomies happen at the level of the LES and cardia as this is the site of narrowing in the submucosal tunnel. If a mucosotomy is identified it should be closed with endoscopic clips. Larger mucosotomies have been closed with a flexible endoscopic suturing device (OverStitch; Apollo Endosurgery, Austin, TX, United States)[56,57]. Other salvage techniques used have included fibrin glue[58] and over-the-scope clips[43]. If the mucosotomy is detected during submucosal tunneling then it should be addressed immediately as delayed closure may result in significant increase is the size of the mucostomy (Figure 13).
Figure 13.
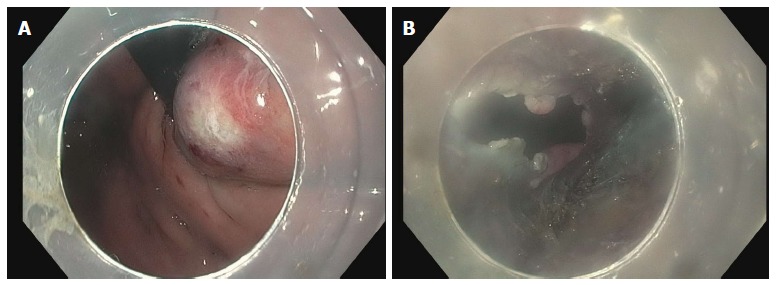
Inadvertent mucosotomy at the gastric cardia. A: Coagulation injury seen on the gastric mucosa at the level of the cardia during the process of submucosal tunneling; B: The procedure continued, however, on completion of the myotomy a frank 12 mm mucosotomy was now present.
Bleeding during submucosal tunneling is not uncommon although the need for specialized interventions is rare (Figure 14). Careful step-wise dissection will allow vessels to be visualized and prophylactically treated using coagulation with the electrocautery knife itself (forced coagulation 25 W effect 2) or hemostatic forceps (Coagrasper; Olympus, Center Valley, PA, United States) for treatment of bigger vessels that are usually encountered in the gastric cardia. If bleeding appears to originate from a vessel along the mucosal surface, hemostasis can be achieved with gentle pressure using the tip of the endoscope for several minutes.
Figure 14.
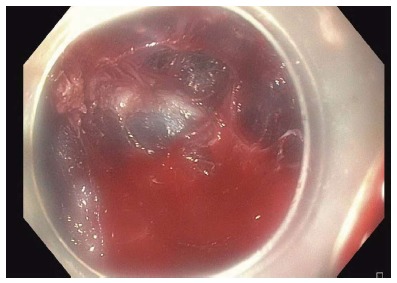
Bleeding encountered during gastric myotomy. This was treated with the use of coagulation graspers.
Late advers events
Delayed bleeding has been reported in 0.7% of patients in a large series of 428 patients[59]. Hematemesis with or without chest pain requires an emergent endoscopy and removal of the clips or sutures from the mucosal entry so that the submucosal tunnel and muscle can be assessed. In the aforementioned series, the bleeding point was identified in 2/3 cases and in the third patient there was no focus found and the patient was effectively treated with a Sengstaken-Blackmore tube. It is important to note that hematoma in the tunnel can result in pressure necrosis of the mucosal flap with potentially disastrous consequences. The safety of the Sengstaken-Blackmore tube in this setting is also debated as it can potentially result in pressure necrosis of the already devascularized mucosa.
The most common adverse event with POEM is gastroesophageal reflux (GER). When objective data are reviewed, such as erosive esophagitis on EGD and/or an abnormal acid exposure on a pH study, the prevalence of GER appears to be between 20% to 46%[6]. This is similar to the rates seen with Heller myotomy with partial fundoplication[60,61]. There is no consensus on how to manage patients with objective GER. One center reported the use of transoral endoscopic fundoplication in an patient with GER symptoms refractory to proton pump inhibitor[62].
POEM IN THE MANAGEMENT ALGORITHM FOR ACHALASIA
For decades, pneumatic balloon dilation and Heller myotomy were the primary methods for the palliation of symptoms of achalasia. POEM appears to have potential advantages over these techniques. POEM appears to be associated with decreased need for retreatment and a lower rate of perforation than pneumatic balloon dilation although no comparative studies exist. There are several uncontrolled studies comparing POEM to Heller myotomy which reveal that they have similar short term efficacy and safety[47,63-65]. Aside from POEM, insertion of self-expandable metallic stents across the EGJ have been studied as an alternative management strategy. Although early reports show promise in terms of symptom palliation, stent migration and intolerance appear to be an issue[66-68].
At this stage, there are no gastrointestinal or surgical society guidelines that have incorporated POEM into their treatment algorithm for achalasia. The American College of Gastroenterology clinical guideline on the management of achalasia cautioned against the use of POEM until the results from further clinical studies are available[69]. Similarly, Vela et al[70] in his expert review, comments that POEM should only be performed in the context of clinical trials and that more data is needed before it can be incorporated into the treatment algorithm for achalasia patients. As POEM disseminates worldwide, it is inevitable that it will establish its place in management algorithms of the future.
GASTRIC PERORAL ENDOSCOPIC MYOTOMY (ENDOSCOPIC PYLOROMYOTOMY)
We have published the first human endoscopic pyloromyotomy for medication refractory gastroparesis[22]. One year later Shlomovitz et al[23] reported gastric peroral endoscopic myotomy in 7 patients with gastroparesis. Six of the 7 patients experienced significant improvement in symptoms with normalization of gastric emptying seen in 4 out of 5 patients. This procedure appears viable and can be executed using similar techniques to that of esophageal POEM. It should be noted that only a subset of patients with refractory gastroparesis are likely to benefit from this approach.
COMMENCING A POEM PROGRAM
It is general consensus that institutions commencing a POEM program do it with institutional review board approval[36]. POEM is a technique that requires a unique set of skills combining good endoscopic manipulation and recognition of anatomical structures. In particular, knowledge of the EGJ anatomy and pathophysiology of achalasia is necessary. Of significant importance, is the maintenance of a consistent team throughout the learning curve. This includes nursing as well as anesthetic staff. Additionally, knowledge of the use of accessories necessary to deal with complications is mandatory. Expert operators propose that an efficient training method involves careful observation of POEM by an expert, experience using live animal models and then performing the procedure in humans with a proctor present.
The learning curve for POEM has not been clearly defined. Kurian et al[71] used the duration of the procedure per centimeter of myotomy and the incidence of inadvertent mucosotomies and calculated that the learning curve appeared to plateau at approximately 20 procedures. Teitelbaum et al[72] found that reduction in time for the mucosal entry and myotomy as well as reduction in the incidence of inadvertent mucosotomies occurred at a “learning rate” of 7 procedures. Expert operators comment that the creation of the submucosal tunnel, particularly at the LES, is likely the most difficult aspect of POEM.
FUTURE DIRECTIONS
Given that achalasia is a chronic disease, long-term outcomes for POEM are essential. Furthermore, POEM needs to be compared to Heller myotomy and pneumatic balloon dilation in multicenter prospective randomized controlled trials.
Progress is being made to simplify POEM in order to increase its efficiency and safety. We published our experience of a novel technique of “auto-tunneling” during POEM in 5 pigs[73]. After creation of the submucosal bleb at the site of the mucosal entry, a proprietary submucosal lifting gel (Cook Medical, Winston-Salem, NC, United States) was injected and resulted in a complete submucosal tunnel to the level of the EGJ. This and other innovative modifications may alleviate current technical challenges.
The most contentious issue surrounding POEM remains the frequency and clinical importance of gastroesophageal reflux. Rigorous evaluations of patients post POEM using pH measurements are required in patients from the East and West. Individualizing the length of the gastric myotomy based on the results of EndoFLIP may reduce the incidence of this problem. Additionally, performing a transoral partial fundoplication in all patients or those that have abnormal acid exposure on post procedure testing may improve outcomes.
Further investigation needs to be performed to study other technical considerations for POEM. Is an anterior or posterior myotomy the preferred approach? Is it cost-effective and safe for POEM to be performed in the endoscopy unit? Is same day discharge suitable if an immediate contrast esophagram demonstrates no extravasation? These, as well as other questions, will need to be answered by high quality controlled trials.
CONCLUSION
POEM likely represents the first sentinel application in NOTES and has the potential to supplant the current treatment methods for achalasia. It fulfils an important clinical need as both pneumatic balloon dilation and Heller myotomy have their short comings. POEM is an elegant minimally invasive treatment with a short-term clinical response of 82% to 100% and with a low risk of adverse events. The results of prospective multicenter randomized controlled trials are awaited.
Footnotes
Conflict-of-interest: Mouen A Khashab is a consultant for Boston Scientific and Olympus America and has received research support from Cook Medical; Vivek Kumbhari has no relevant disclosures.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 6, 2014
First decision: October 28, 2014
Article in press: January 20, 2015
P- Reviewer: Albuquerque A, Fabre JM, Luo HS, Matsumoto S S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
References
- 1.Ortega JA, Madureri V, Perez L. Endoscopic myotomy in the treatment of achalasia. Gastrointest Endosc. 1980;26:8–10. doi: 10.1016/s0016-5107(80)73249-2. [DOI] [PubMed] [Google Scholar]
- 2.Khashab MA, Kalloo AN. NOTES: current status and new horizons. Gastroenterology. 2012;142:704–710.e1. doi: 10.1053/j.gastro.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Sumiyama K, Gostout CJ, Rajan E, Bakken TA, Knipschield MA, Marler RJ. Submucosal endoscopy with mucosal flap safety valve. Gastrointest Endosc. 2007;65:688–694. doi: 10.1016/j.gie.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Pasricha PJ, Hawari R, Ahmed I, Chen J, Cotton PB, Hawes RH, Kalloo AN, Kantsevoy SV, Gostout CJ. Submucosal endoscopic esophageal myotomy: a novel experimental approach for the treatment of achalasia. Endoscopy. 2007;39:761–764. doi: 10.1055/s-2007-966764. [DOI] [PubMed] [Google Scholar]
- 5.Inoue H, Minami H, Kobayashi Y, Sato Y, Kaga M, Suzuki M, Satodate H, Odaka N, Itoh H, Kudo S. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265–271. doi: 10.1055/s-0029-1244080. [DOI] [PubMed] [Google Scholar]
- 6.Stavropoulos SN, Desilets DJ, Fuchs KH, Gostout CJ, Haber G, Inoue H, Kochman ML, Modayil R, Savides T, Scott DJ, et al. Per-oral endoscopic myotomy white paper summary. Gastrointest Endosc. 2014;80:1–15. doi: 10.1016/j.gie.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Onimaru M, Inoue H, Ikeda H, Yoshida A, Santi EG, Sato H, Ito H, Maselli R, Kudo SE. Peroral endoscopic myotomy is a viable option for failed surgical esophagocardiomyotomy instead of redo surgical Heller myotomy: a single center prospective study. J Am Coll Surg. 2013;217:598–605. doi: 10.1016/j.jamcollsurg.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 8.Zhou PH, Li QL, Yao LQ, Xu MD, Chen WF, Cai MY, Hu JW, Li L, Zhang YQ, Zhong YS, et al. Peroral endoscopic remyotomy for failed Heller myotomy: a prospective single-center study. Endoscopy. 2013;45:161–166. doi: 10.1055/s-0032-1326203. [DOI] [PubMed] [Google Scholar]
- 9.Vigneswaran Y, Yetasook AK, Zhao JC, Denham W, Linn JG, Ujiki MB. Peroral endoscopic myotomy (POEM): feasible as reoperation following Heller myotomy. J Gastrointest Surg. 2014;18:1071–1076. doi: 10.1007/s11605-014-2496-2. [DOI] [PubMed] [Google Scholar]
- 10.Yang D, Draganov PV. Peroral endoscopic myotomy (POEM) for achalasia after Roux-en-Y gastric bypass. Endoscopy. 2014;46 Suppl 1 UCTN:E11–E12. doi: 10.1055/s-0033-1359140. [DOI] [PubMed] [Google Scholar]
- 11.Inoue H, Tianle KM, Ikeda H, Hosoya T, Onimaru M, Yoshida A, Minami H, Kudo SE. Peroral endoscopic myotomy for esophageal achalasia: technique, indication, and outcomes. Thorac Surg Clin. 2011;21:519–525. doi: 10.1016/j.thorsurg.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Chen WF, Li QL, Zhou PH, Yao LQ, Xu MD, Zhang YQ, Zhong YS, Ma LL, Qin WZ, Hu JW, et al. Long-term outcomes of peroral endoscopic myotomy for achalasia in pediatric patients: a prospective, single-center study. Gastrointest Endosc. 2015;81:91–100. doi: 10.1016/j.gie.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 13.Familiari P, Marchese M, Gigante G, Boskoski I, Tringali A, Perri V, Costamagna G. Peroral endoscopic myotomy for the treatment of achalasia in children. J Pediatr Gastroenterol Nutr. 2013;57:794–797. doi: 10.1097/MPG.0b013e3182a803f7. [DOI] [PubMed] [Google Scholar]
- 14.Kandulski A, Fuchs KH, Weigt J, Malfertheiner P. Jackhammer esophagus: high-resolution manometry and therapeutic approach using peroral endoscopic myotomy (POEM) Dis Esophagus. 2014:Jan 27; Epub ahead of print. doi: 10.1111/dote.12182. [DOI] [PubMed] [Google Scholar]
- 15.Khashab MA, Saxena P, Kumbhari V, Nandwani M, Roland BC, Stein E, Clarke JO, Stavropoulos S, Inoue H, Pasricha PJ. Peroral endoscopic myotomy as a platform for the treatment of spastic esophageal disorders refractory to medical therapy (with video) Gastrointest Endosc. 2014;79:136–139. doi: 10.1016/j.gie.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 16.Kristensen HØ, Bjerregaard NC, Rask P, Mortensen FV, Kunda R. Peroral endoscopic myotomy (POEM) for nutcracker esophagus. Three cases with 12 months follow-up. Scand J Gastroenterol. 2014;49:1285–1289. doi: 10.3109/00365521.2014.958096. [DOI] [PubMed] [Google Scholar]
- 17.Louis H, Covas A, Coppens E, Devière J. Distal esophageal spasm treated by peroral endoscopic myotomy. Am J Gastroenterol. 2012;107:1926–1927. doi: 10.1038/ajg.2012.317. [DOI] [PubMed] [Google Scholar]
- 18.Minami H, Isomoto H, Yamaguchi N, Ohnita K, Takeshima F, Inoue H, Nakao K. Peroral endoscopic myotomy (POEM) for diffuse esophageal spasm. Endoscopy. 2014;46 Suppl 1 UCTN:E79–E81. doi: 10.1055/s-0032-1309922. [DOI] [PubMed] [Google Scholar]
- 19.Shiwaku H, Inoue H, Beppu R, Nakashima R, Minami H, Shiroshita T, Yamauchi Y, Hoshino S, Yamashita Y. Successful treatment of diffuse esophageal spasm by peroral endoscopic myotomy. Gastrointest Endosc. 2013;77:149–150. doi: 10.1016/j.gie.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Roman S, Kahrilas PJ. Management of spastic disorders of the esophagus. Gastroenterol Clin North Am. 2013;42:27–43. doi: 10.1016/j.gtc.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khashab MA, Messallam AA, Onimaru M, Teitelbaum EN, Ujiki MB, Gitelis ME, Modayil RJ, Hungness ES, Stavropoulos SN, El Zein MH, et al. International Multicenter Experience with Peroral Endoscopic Myotomy (POEM) for the Treatment of Spastic Esophageal Disorders Refractory to Medical Therapy. Gastrointestinal Endoscopy. 2014;79 Suppl:AB167. doi: 10.1016/j.gie.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Khashab MA, Stein E, Clarke JO, Saxena P, Kumbhari V, Chander Roland B, Kalloo AN, Stavropoulos S, Pasricha P, Inoue H. Gastric peroral endoscopic myotomy for refractory gastroparesis: first human endoscopic pyloromyotomy (with video) Gastrointest Endosc. 2013;78:764–768. doi: 10.1016/j.gie.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 23.Shlomovitz E, Pescarus R, Cassera MA, Sharata AM, Reavis KM, Dunst CM, Swanström LL. Early human experience with per-oral endoscopic pyloromyotomy (POP) Surg Endosc. 2015;29:543–551. doi: 10.1007/s00464-014-3720-6. [DOI] [PubMed] [Google Scholar]
- 24.Eckardt VF. Clinical presentations and complications of achalasia. Gastrointest Endosc Clin N Am. 2001;11:281–292, vi. [PubMed] [Google Scholar]
- 25.Greene CL, Chang EJ, Oh DS, Worrell SG, Hagen JA, DeMeester SR. High resolution manometry sub-classification of Achalasia: does it really matter? : Does Achalasia sub-classification matter? Surg Endosc. 2014:Sep 24; Epub ahead of print. doi: 10.1007/s00464-014-3804-3. [DOI] [PubMed] [Google Scholar]
- 26.Pescarus R, Shlomovitz E, Swanstrom LL. Per-oral endoscopic myotomy (POEM) for esophageal achalasia. Curr Gastroenterol Rep. 2014;16:369. doi: 10.1007/s11894-013-0369-6. [DOI] [PubMed] [Google Scholar]
- 27.Saxena P, Pippenger R, Khashab MA. Preventing aspiration during peroral endoscopic myotomy. J Anesth. 2014;28:959. doi: 10.1007/s00540-014-1898-3. [DOI] [PubMed] [Google Scholar]
- 28.Teitelbaum EN, Soper NJ, Pandolfino JE, Kahrilas PJ, Hirano I, Boris L, Nicodème F, Lin Z, Hungness ES. Esophagogastric junction distensibility measurements during Heller myotomy and POEM for achalasia predict postoperative symptomatic outcomes. Surg Endosc. 2015;29:522–528. doi: 10.1007/s00464-014-3733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rieder E, Swanström LL, Perretta S, Lenglinger J, Riegler M, Dunst CM. Intraoperative assessment of esophagogastric junction distensibility during per oral endoscopic myotomy (POEM) for esophageal motility disorders. Surg Endosc. 2013;27:400–405. doi: 10.1007/s00464-012-2484-0. [DOI] [PubMed] [Google Scholar]
- 30.Teitelbaum EN, Soper NJ, Pandolfino JE, Kahrilas PJ, Boris L, Nicodème F, Lin Z, Hungness ES. An extended proximal esophageal myotomy is necessary to normalize EGJ distensibility during Heller myotomy for achalasia, but not POEM. Surg Endosc. 2014;28:2840–2847. doi: 10.1007/s00464-014-3563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khashab MA, Messallam AA, Saxena P, Kumbhari V, Ricourt E, Aguila G, Roland BC, Stein E, Nandwani M, Inoue H, et al. Jet injection of dyed saline facilitates efficient peroral endoscopic myotomy. Endoscopy. 2014;46:298–301. doi: 10.1055/s-0033-1359024. [DOI] [PubMed] [Google Scholar]
- 32.Khashab MA, Kumbhari V, Kalloo AN, Saxena P. Peroral endoscopic myotomy: a 4-step approach to a challenging procedure. Gastrointest Endosc. 2014;79:997–998. doi: 10.1016/j.gie.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Cai MY, Zhou PH, Yao LQ, Xu MD, Zhong YS, Li QL, Chen WF, Hu JW, Cui Z, Zhu BQ. Peroral endoscopic myotomy for idiopathic achalasia: randomized comparison of water-jet assisted versus conventional dissection technique. Surg Endosc. 2014;28:1158–1165. doi: 10.1007/s00464-013-3300-1. [DOI] [PubMed] [Google Scholar]
- 34.Litle VR. Laparoscopic Heller myotomy for achalasia: a review of the controversies. Ann Thorac Surg. 2008;85:S743–S746. doi: 10.1016/j.athoracsur.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Carter JT, Nguyen D, Roll GR, Ma SW, Way LW. Predictors of long-term outcome after laparoscopic esophagomyotomy and Dor fundoplication for achalasia. Arch Surg. 2011;146:1024–1028. doi: 10.1001/archsurg.2011.214. [DOI] [PubMed] [Google Scholar]
- 36.Stavropoulos SN, Modayil RJ, Friedel D, Savides T. The International Per Oral Endoscopic Myotomy Survey (IPOEMS): a snapshot of the global POEM experience. Surg Endosc. 2013;27:3322–3338. doi: 10.1007/s00464-013-2913-8. [DOI] [PubMed] [Google Scholar]
- 37.Minami H, Inoue H, Haji A, Isomoto H, Urabe S, Hashiguchi K, Matsushima K, Akazawa Y, Yamaguchi N, Ohnita K, et al. Per-oral endoscopic myotomy: emerging indications and evolving techniques. Dig Endosc. 2015;27:175–181. doi: 10.1111/den.12328. [DOI] [PubMed] [Google Scholar]
- 38.Baldaque-Silva F, Marques M, Vilas-Boas F, Maia JD, Sá F, Macedo G. New transillumination auxiliary technique for peroral endoscopic myotomy. Gastrointest Endosc. 2014;79:544–545. doi: 10.1016/j.gie.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 39.Kumbhari V, Saxena P, Messallam AA, Aguila G, Tieu AH, El-Zein M, Kalloo AN, Khashab MA. Fluoroscopy to document the extent of cardiomyotomy during peroral endoscopic myotomy. Endoscopy. 2014;46 Suppl 1 UCTN:E369–E370. doi: 10.1055/s-0034-1377353. [DOI] [PubMed] [Google Scholar]
- 40.Li QL, Chen WF, Zhou PH, Yao LQ, Xu MD, Hu JW, Cai MY, Zhang YQ, Qin WZ, Ren Z. Peroral endoscopic myotomy for the treatment of achalasia: a clinical comparative study of endoscopic full-thickness and circular muscle myotomy. J Am Coll Surg. 2013;217:442–451. doi: 10.1016/j.jamcollsurg.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 41.Von Renteln D, Fuchs KH, Fockens P, Bauerfeind P, Vassiliou MC, Werner YB, Fried G, Breithaupt W, Heinrich H, Bredenoord AJ, et al. Peroral endoscopic myotomy for the treatment of achalasia: an international prospective multicenter study. Gastroenterology. 2013;145:309–11.e1-3. doi: 10.1053/j.gastro.2013.04.057. [DOI] [PubMed] [Google Scholar]
- 42.Sharata AM, Dunst CM, Pescarus R, Shlomovitz E, Wille AJ, Reavis KM, Swanström LL. Peroral endoscopic myotomy (POEM) for esophageal primary motility disorders: analysis of 100 consecutive patients. J Gastrointest Surg. 2015;19:161–170; discussion 170. doi: 10.1007/s11605-014-2610-5. [DOI] [PubMed] [Google Scholar]
- 43.Kumbhari V, Azola A, Saxena P, Modayil R, Kalloo AN, Stavropoulos SN, Khashab MA. Closure methods in submucosal endoscopy. Gastrointest Endosc. 2014;80:894–895. doi: 10.1016/j.gie.2014.01.048. [DOI] [PubMed] [Google Scholar]
- 44.Saxena P, Chavez YH, Kord Valeshabad A, Kalloo AN, Khashab MA. An alternative method for mucosal flap closure during peroral endoscopic myotomy using an over-the-scope clipping device. Endoscopy. 2013;45:579–581. doi: 10.1055/s-0032-1326398. [DOI] [PubMed] [Google Scholar]
- 45.Ling T, Pei Q, Pan J, Zhang X, Lv Y, Li W, Zou X. Successful use of a covered, retrievable stent to seal a ruptured mucosal flap safety valve during peroral endoscopic myotomy in a child with achalasia. Endoscopy. 2013;45 Suppl 2 UCTN:E63–E64. doi: 10.1055/s-0032-1325977. [DOI] [PubMed] [Google Scholar]
- 46.Cai MY, Zhou PH, Yao LQ, Zhu BQ, Liang L, Li QL. Thoracic CT after peroral endoscopic myotomy for the treatment of achalasia. Gastrointest Endosc. 2014;80:1046–1055. doi: 10.1016/j.gie.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Teitelbaum EN, Rajeswaran S, Zhang R, Sieberg RT, Miller FH, Soper NJ, Hungness ES. Peroral esophageal myotomy (POEM) and laparoscopic Heller myotomy produce a similar short-term anatomic and functional effect. Surgery. 2013;154:885–891; discussion 891-892. doi: 10.1016/j.surg.2013.04.051. [DOI] [PubMed] [Google Scholar]
- 48.Verlaan T, Rohof WO, Bredenoord AJ, Eberl S, Rösch T, Fockens P. Effect of peroral endoscopic myotomy on esophagogastric junction physiology in patients with achalasia. Gastrointest Endosc. 2013;78:39–44. doi: 10.1016/j.gie.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Vigneswaran Y, Tanaka R, Gitelis M, Carbray J, Ujiki MB. Quality of life assessment after peroral endoscopic myotomy. Surg Endosc. 2014:Sep 24; Epub ahead of print. doi: 10.1007/s00464-014-3793-2. [DOI] [PubMed] [Google Scholar]
- 50.Rohof WO, Hirsch DP, Kessing BF, Boeckxstaens GE. Efficacy of treatment for patients with achalasia depends on the distensibility of the esophagogastric junction. Gastroenterology. 2012;143:328–335. doi: 10.1053/j.gastro.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 51.Sharata A, Kurian AA, Dunst CM, Bhayani NH, Reavis KM, Swanström LL. Peroral endoscopic myotomy (POEM) is safe and effective in the setting of prior endoscopic intervention. J Gastrointest Surg. 2013;17:1188–1192. doi: 10.1007/s11605-013-2193-6. [DOI] [PubMed] [Google Scholar]
- 52.Ling T, Guo H, Zou X. Effect of peroral endoscopic myotomy in achalasia patients with failure of prior pneumatic dilation: a prospective case-control study. J Gastroenterol Hepatol. 2014;29:1609–1613. doi: 10.1111/jgh.12570. [DOI] [PubMed] [Google Scholar]
- 53.Orenstein SB, Raigani S, Wu YV, Pauli EM, Phillips MS, Ponsky JL, Marks JM. Peroral endoscopic myotomy (POEM) leads to similar results in patients with and without prior endoscopic or surgical therapy. Surg Endosc. 2014:Sep 24; Epub ahead of print. doi: 10.1007/s00464-014-3782-5. [DOI] [PubMed] [Google Scholar]
- 54.Boeckxstaens GE, Annese V, des Varannes SB, Chaussade S, Costantini M, Cuttitta A, Elizalde JI, Fumagalli U, Gaudric M, Rohof WO, et al. Pneumatic dilation versus laparoscopic Heller’s myotomy for idiopathic achalasia. N Engl J Med. 2011;364:1807–1816. doi: 10.1056/NEJMoa1010502. [DOI] [PubMed] [Google Scholar]
- 55.Swanstrom LL, Kurian A, Dunst CM, Sharata A, Bhayani N, Rieder E. Long-term outcomes of an endoscopic myotomy for achalasia: the POEM procedure. Ann Surg. 2012;256:659–667. doi: 10.1097/SLA.0b013e31826b5212. [DOI] [PubMed] [Google Scholar]
- 56.Kurian AA, Bhayani NH, Reavis K, Dunst C, Swanström L. Endoscopic suture repair of full-thickness esophagotomy during per-oral esophageal myotomy for achalasia. Surg Endosc. 2013;27:3910. doi: 10.1007/s00464-013-3002-8. [DOI] [PubMed] [Google Scholar]
- 57.Modayil R, Friedel D, Stavropoulos SN. Endoscopic suture repair of a large mucosal perforation during peroral endoscopic myotomy for treatment of achalasia. Gastrointest Endosc. 2014;80:1169–1170. doi: 10.1016/j.gie.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 58.Li H, Linghu E, Wang X. Fibrin sealant for closure of mucosal penetration at the cardia during peroral endoscopic myotomy (POEM) Endoscopy. 2012;44 Suppl 2 UCTN:E215–E216. doi: 10.1055/s-0032-1309358. [DOI] [PubMed] [Google Scholar]
- 59.Li QL, Zhou PH, Yao LQ, Xu MD, Chen WF, Hu JW, Cai MY, Zhang YQ, Zhong YS, Qin WZ, et al. Early diagnosis and management of delayed bleeding in the submucosal tunnel after peroral endoscopic myotomy for achalasia (with video) Gastrointest Endosc. 2013;78:370–374. doi: 10.1016/j.gie.2013.04.172. [DOI] [PubMed] [Google Scholar]
- 60.Rawlings A, Soper NJ, Oelschlager B, Swanstrom L, Matthews BD, Pellegrini C, Pierce RA, Pryor A, Martin V, Frisella MM, et al. Laparoscopic Dor versus Toupet fundoplication following Heller myotomy for achalasia: results of a multicenter, prospective, randomized-controlled trial. Surg Endosc. 2012;26:18–26. doi: 10.1007/s00464-011-1822-y. [DOI] [PubMed] [Google Scholar]
- 61.Khajanchee YS, Kanneganti S, Leatherwood AE, Hansen PD, Swanström LL. Laparoscopic Heller myotomy with Toupet fundoplication: outcomes predictors in 121 consecutive patients. Arch Surg. 2005;140:827–833; discussion 833-834. doi: 10.1001/archsurg.140.9.827. [DOI] [PubMed] [Google Scholar]
- 62.Kumta NA, Kedia P, Sethi A, Kahaleh M. Transoral incisionless fundoplication for treatment of refractory GERD after peroral endoscopic myotomy. Gastrointest Endosc. 2015;81:224–225. doi: 10.1016/j.gie.2014.05.321. [DOI] [PubMed] [Google Scholar]
- 63.Hungness ES, Teitelbaum EN, Santos BF, Arafat FO, Pandolfino JE, Kahrilas PJ, Soper NJ. Comparison of perioperative outcomes between peroral esophageal myotomy (POEM) and laparoscopic Heller myotomy. J Gastrointest Surg. 2013;17:228–235. doi: 10.1007/s11605-012-2030-3. [DOI] [PubMed] [Google Scholar]
- 64.Ujiki MB, Yetasook AK, Zapf M, Linn JG, Carbray JM, Denham W. Peroral endoscopic myotomy: A short-term comparison with the standard laparoscopic approach. Surgery. 2013;154:893–897; discussion 897-900. doi: 10.1016/j.surg.2013.04.042. [DOI] [PubMed] [Google Scholar]
- 65.Bhayani NH, Kurian AA, Dunst CM, Sharata AM, Rieder E, Swanstrom LL. A comparative study on comprehensive, objective outcomes of laparoscopic Heller myotomy with per-oral endoscopic myotomy (POEM) for achalasia. Ann Surg. 2014;259:1098–1103. doi: 10.1097/SLA.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 66.Coppola F, Gaia S, Rolle E, Recchia S. Temporary endoscopic metallic stent for idiopathic esophageal achalasia. Surg Innov. 2014;21:11–14. doi: 10.1177/1553350613492024. [DOI] [PubMed] [Google Scholar]
- 67.Zeng Y, Dai YM, Wan XJ. Clinical remission following endoscopic placement of retrievable, fully covered metal stents in patients with esophageal achalasia. Dis Esophagus. 2014;27:103–108. doi: 10.1111/dote.12083. [DOI] [PubMed] [Google Scholar]
- 68.Li YD, Cheng YS, Li MH, Chen NW, Chen WX, Zhao JG. Temporary self-expanding metallic stents and pneumatic dilation for the treatment of achalasia: a prospective study with a long-term follow-up. Dis Esophagus. 2010;23:361–367. doi: 10.1111/j.1442-2050.2010.01048.x. [DOI] [PubMed] [Google Scholar]
- 69.Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol. 2013;108:1238–1249; quiz 1250. doi: 10.1038/ajg.2013.196. [DOI] [PubMed] [Google Scholar]
- 70.Vela MF. Management strategies for achalasia. Neurogastroenterol Motil. 2014;26:1215–1221. doi: 10.1111/nmo.12416. [DOI] [PubMed] [Google Scholar]
- 71.Kurian AA, Dunst CM, Sharata A, Bhayani NH, Reavis KM, Swanström LL. Peroral endoscopic esophageal myotomy: defining the learning curve. Gastrointest Endosc. 2013;77:719–725. doi: 10.1016/j.gie.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 72.Teitelbaum EN, Soper NJ, Arafat FO, Santos BF, Kahrilas PJ, Pandolfino JE, Hungness ES. Analysis of a learning curve and predictors of intraoperative difficulty for peroral esophageal myotomy (POEM) J Gastrointest Surg. 2014;18:92–98; discussion 98-99. doi: 10.1007/s11605-013-2332-0. [DOI] [PubMed] [Google Scholar]
- 73.Khashab MA, Sharaiha RZ, Saxena P, Law JK, Singh VK, Lennon AM, Shin EJ, Canto MI, Aguila G, Okolo PI, et al. Novel technique of auto-tunneling during peroral endoscopic myotomy (with video) Gastrointest Endosc. 2013;77:119–122. doi: 10.1016/j.gie.2012.09.011. [DOI] [PubMed] [Google Scholar]


