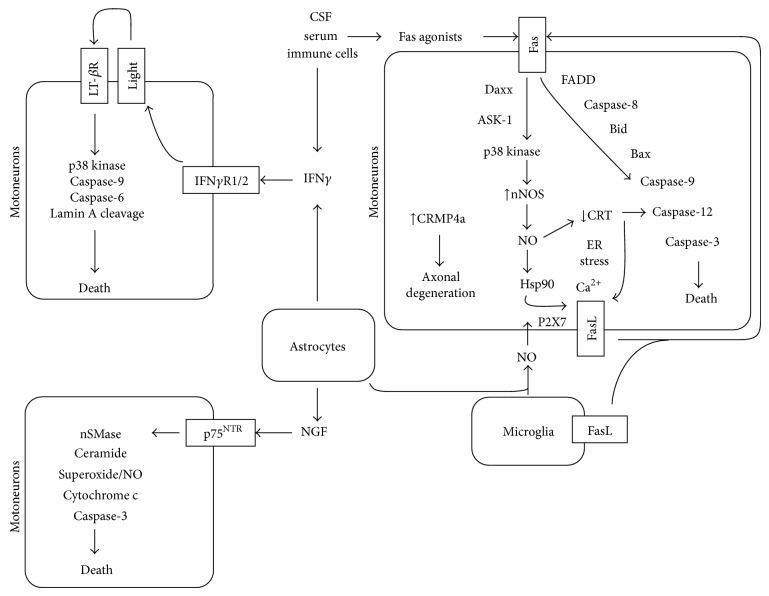
Figure 1.
Active killing of motoneurons through death receptors under pathological context. Fas activation induces a motoneuron-restricted signaling pathway, in which both Fas-Daxx and Fas-FADD branches act synergistically to execute the death program. The production of NO is an obligatory step of the Fas pathway that leads to the downregulation of CRT, which promotes ER stress and upregulation of FasL that in turn activates Fas. Mobilization of FasL to the plasma membrane was also shown to occur following the stimulation of P2X7 receptor by nitrated Hsp90. CRMP4a ALS-linked SOD1 sensitizes motoneurons to this Fas/NO feedback loop. The entry in the amplification loop can be achieved by exogenous NO or Fas agonists (i.e., FasL or circulating agonistic anti-Fas antibodies) that can originate from motoneuron environment. IFNγ produced by mutant astrocytes promotes the engagement of LT-βR pathway by increasing levels of LIGHT in motoneurons. LT-βR-mediated death of motoneurons implicates p38 kinase, caspase-9 and -6, and lamin A cleavage but occurs independently of caspase-8, cytochrome c release, and the caspase-3/-7 pathway. Immune cells, serum, and cerebrospinal fluid may represent another source for IFNγ. NGF produced by activated astrocytes triggers death of motoneurons following engagement of p75NTR, which become reexpressed in pathological condition. p75NTR-induced death of motoneurons involves activation of nSMase, production of ceramide, formation of reactive oxygen and nitrogen species, the release of cytochrome c from the mitochondria, and activation of caspase-3. Regarding their own proper characteristics, vulnerable motoneurons would be more susceptible to this non-cell-autonomous effect.