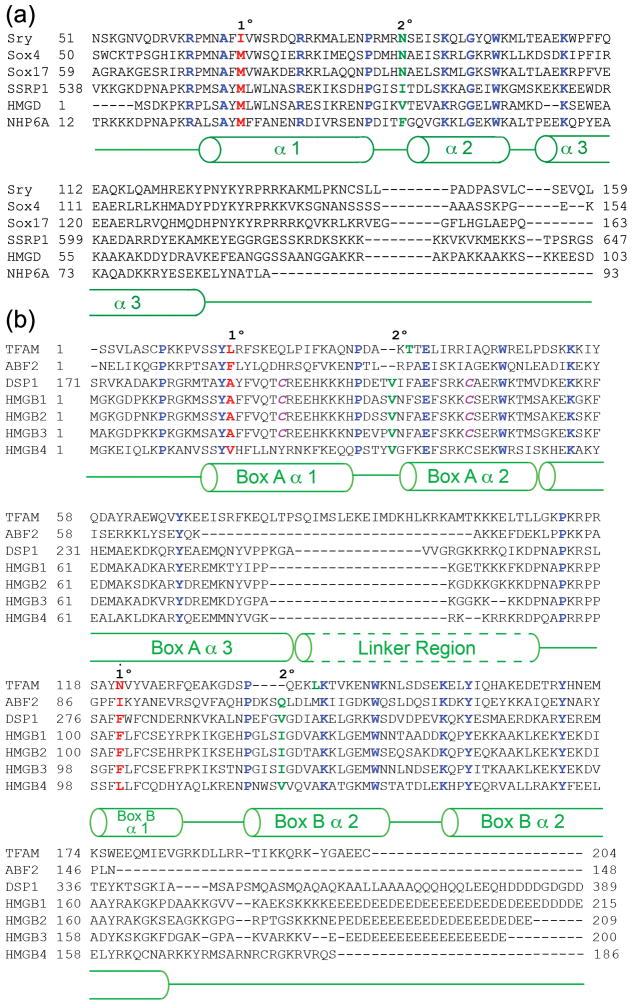
Figure 1. Alignments of single- and dual-domain high mobility group (HMG)-box proteins.
(a) Sequence alignment of the HMG domains of selected single HMG box proteins: structure specific recognition protein 1 (SSRP1), sex-determining region Y (SRY) [80], and the SRY-like box (Sox) family of HMGB proteins [81]. Primary site (1°, red font) intercalating residues and secondary site (‘2°’, green font) intercalating/hydrogen bonding residues are indicated. Conserved residues are shown in blue font. Green cylinders below the sequence alignment illustrate the regions of the proteins that form the alpha helices of the HMG box. (b) Sequence alignment of the HMG domains of selected dual HMG box proteins including mitochondrial transcription factor A (TFAM); the Saccharomyces cerevisiae homologue of TFAM, ABF2 (*numbering here begins after the 42 or 35 amino acid residue mitochondrial localization sequence for TFAM or ABF2, respectively); the Drosophila melanogaster dorsal repressor DSP1, and HMGB1-4. Primary and secondary intercalating residues and conserved residues are indicated as in (a). The cysteine residues that have been shown to form an intramolecular disulfide bond in HMGB1 are shown in magenta. The green unbroken cylinders below the sequence alignment indicate the amino acids that form the helical structures comprising the HMG boxes of TFAM, when bound to DNA. The broken green cylinder represents the linker region of TFAM that forms an alpha helix upon binding to DNA.