Fig 2. Cupin motifs and metal binding residues of DddW.
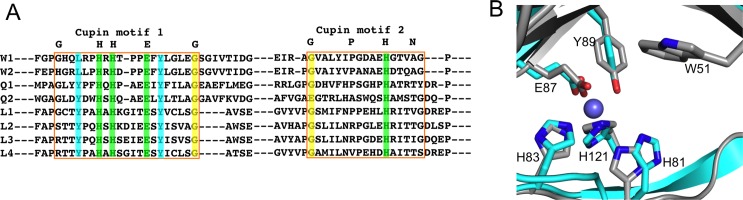
(A) Sequence alignment of cupin regions of selected DddW, DddQ and DddL proteins using sequences deposited at NCBI and CLUSTAL 2.1 for the alignment. The two conserved cupin motifs 1 (GX5HXHX3,4EX6G) and 2 (GX5PXGX2HX3N), containing residues that bind metal ions and are catalytically important are highlighted in green. Tyr residues playing catalytic role in Ruegeria lacuscaerulensis DddQ are marked cyan and other conserved residues in the cupin motifs are colored yellow. The sequences are from: W1 = DddW, Ruegeria pomeroyi DSS-3 (SPO0453); W2 = DddW, Roseobacter sp. MED193, (MED193_09710); Q1 = DddQ, Ruegeria pomeroyi DSS-3 (SPO1596); Q2 = DddQ, Ruegeria lacuscaerulensis (ITI-1157); L1 = DddL, Sulfitobacter sp. EE-36 (EE36_11918); L2 = DddL, Rhodobacter sphaeroides 2.4.1 (RSP_1433); L3 = DddL, Roseibacterium elongatum DSM 19469 (roselon_02436); L4 = DddL, Caenispirillum salinarum (C882_2645). (B) Homology model of Ruegeria pomeroyi DddW (grey) (generated using Phyre 2 [52]) superimposed on the Zn(II)-bound structure of Ruegeria lacuscaerulensis DddQ (cyan) (PDB 4LA2). The homology model of DddW shows the catalytic residues H81, H83, E87, and H121. Most of these residues of DddW (H83, E87, and H121) superimpose well on the zinc-coordinating DddQ residues (H125, E129, and H163). While Tyr usually is not involved in metal ion binding in cupin proteins, the DddQ structure shows a Zn-coordinated Tyr residue (Tyr131) and this Tyr superimposes on Tyr89 of DddW. The side chain residues are shown in ball and stick with oxygens in red, nitrogens in blue, zinc in slate, and carbons are similar to protein backbone.
