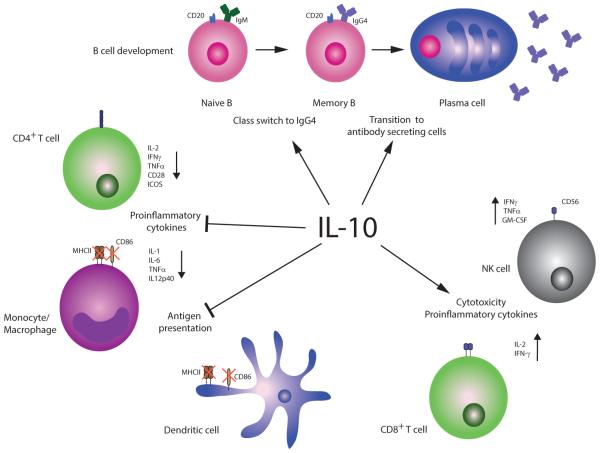
Figure 1. IL-10 in general immune homeostasis.
IL-10 inhibits proinflammatory cytokine production, including IL-2, interferon gamma (IFNγ), and tumor necrosis factor alpha (TNFα) from CD4+ T cells [7], as well as IL-1, IL-6, TNFα, and IL-12 production from monocytes and macrophages [8, 9]. IL-10 impairs CD4+ T helper cell effector and memory responses by inhibiting CD28 and inducible costimulator (ICOS) T cell signaling [10] and downregulating major histocompatibility complex class II (MHCII) and CD86 costimulatory molecules on monocytes and dendritic cells, which prevents effective antigen presentation [11-13]. IL-10 expression in B cells favors class switch to IgG4 [14] and B cell differentiation into plasma cells [31], which can have anti-inflammatory effects due to IgG4’s inability to fix complement or form immune complexes. Alongside these predominantly anti-inflammatory functions, IL-10 (in conjunction with IL-2) can increase cytotoxicity of CD8+ T cells [16] and natural killer cells [18] by upregulation of pro-inflammatory cytokines, including IFNγ, IL-2, TNFα, and/or GM-CSF. However, long-term exposure of CD4+ and CD8+ T cells to IL-10 can result in T cell exhaustion [81, 82], including lack of cytotoxicity, cytokine production, and antigen-induced proliferation.