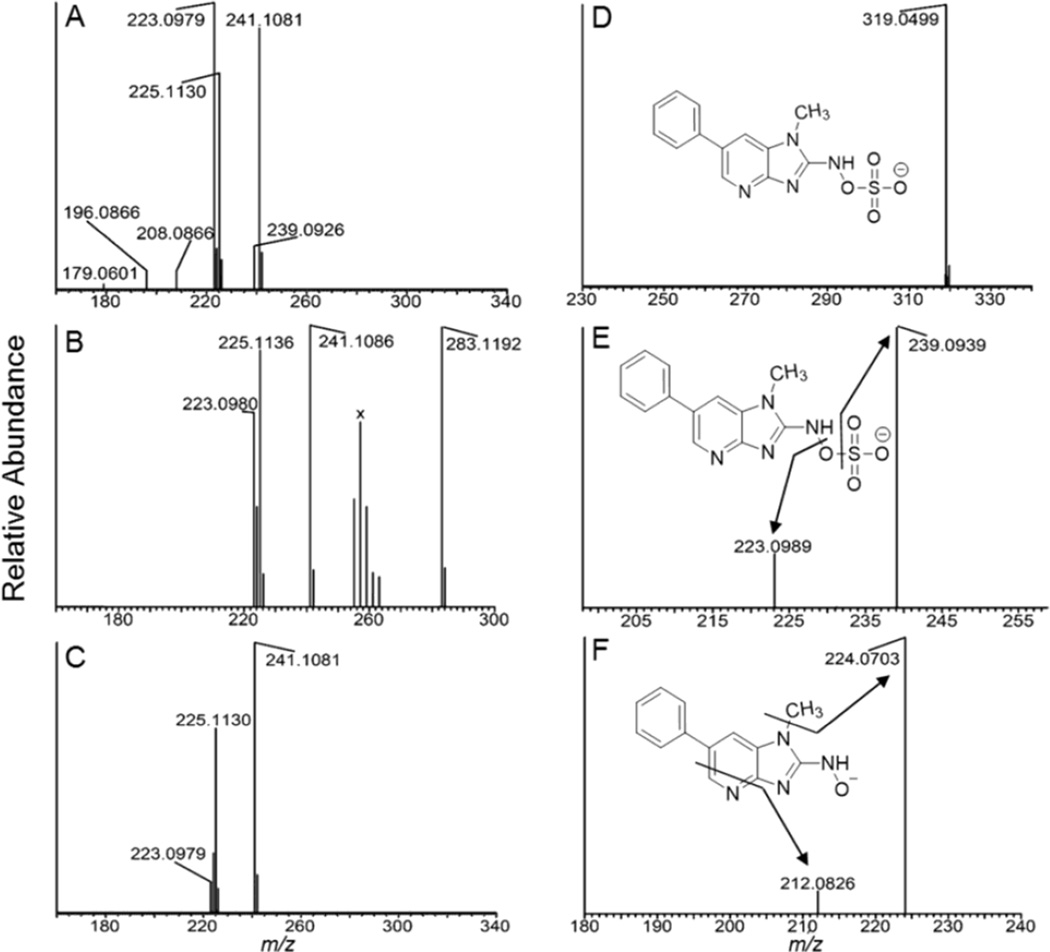
Figure 2.
(A) ESI-MS full scan mass spectra of (A) N-sulfooxy-PhIP(m/z 321.0652), (B) N-acetoxy-PhIP (m/z 283.1190), and (C) HONH-PhIP (m/z 241.1084) in the positive ion mode. The ion observed at m/z 225.11130 is attributed to PhIP and occurs by in-source reduction of HONH-PhIP. (D) Product ion spectrum of N-sulfooxy-PhIP ([M-H]m/z 319.0506 acquired at 0 collision energy, isolation width m/z 1). (E) Product ion spectra of N-sulfooxy-PhIP ion at m/z 319.1; and (F) product ion spectra of N-sulfooxy-PhIP at the MS3 scan stage (319.0 > 239.1 >) in the negative ion mode. The cluster of ions designated X between m/z 255 – 263 in the full scan mass spectrum of N-acetoxy-PhIP (B) are background ions present in the reaction medium.