Abstract
Human and nonhuman primates are not mentally constrained to the present. They can remember the past and – at least to an extent – anticipate the future. Anticipation of the future ranges from long-term prospection such as planning for retirement to more short-term future oriented cognition such as planning a route through a maze. Here we tested a great ape species (chimpanzees), an Old World monkey species (rhesus macaques) a New World monkey species (capuchin monkeys) and human children on a computerized maze task. All subjects had to move a cursor through a maze to reach a goal at the bottom of the screen. For best performance on the task, subjects had to “plan ahead” to the end of the maze to move the cursor in the correct direction, avoid traps, and reverse directions if necessary. Mazes varied in difficulty. Chimpanzees were better than both monkey species, and monkeys showed a particular deficit when moving away from the goal or changing directions was required. Children showed a similar pattern to monkeys regarding the effects of reversals and moves away from the goal, but their overall performance in terms of correct maze completion was similar to the chimpanzees. The results highlight similarities as well as differences in planning across species and the role that inhibitory control may play in future oriented cognition in primates.
Keywords: Planning, Mazes, Chimpanzees, Rhesus Monkeys, Capuchin Monkeys, Children, Future Oriented Processes
There is great interest in comparative cognitive science about the ability of nonhuman animals to remember their own past and perhaps to plan for their own future (Crystal, 2013; Roberts, 2002; Suddendorf & Busby, 2003; Suddendorf & Corballis, 2007; Tulving, 2005). For adult humans, the most striking examples of these cognitive processes include episodic or autobiographical memory (Tulving, 1972, 1993). Future-oriented processes in humans include instances of prospective memory (i.e., remembering to do something later; McDaniel & Einstein, 2007), but also more episodic-like instances such as imagining one’s own future wedding or retirement. There is now some evidence of episodic-like memory for past events in animals (e.g., Babb & Crystal, 2005; Clayton & Dickinson, 1998, 1999; Eacott, Easton, & Zinkivskay, 2008; Menzel, 1999; Zentall, 2005), and some evidence for more future-oriented processes in nonhuman animals as well (e.g., Beran, Perdue, Bramlett, Menzel, & Evans, 2012; Chappell & Kacelnik, 2002; Clayton, Dally, Gilbert, & Dickinson, 2005; Mulcahy & Call, 2006; Naqshbandi & Roberts 2006; Osvath & Osvath, 2008; Raby, Alexis, Dickinson, & Clayton, 2007; Wilson & Crystal, 2012, Wilson, Pizzo, & Crystal, 2013; Zentall, 2005).
In addition to studying temporally extended retrospection or prospection, studying instances of planning, even those that occur on very limited time scales, can provide insights about the comparative foundations of future-oriented cognition as well as the potential limitations faced by species other than humans in such future orientation. Broadly defined, planning simply requires the organization of behavior in the present to obtain a future goal. The time frame does not matter, and so planning can occur for an event that is seconds, hours, days, or years in the future (Miller, Galanter, & Pribram 1986). Of course, the time scale and spatial scale can be important aspects in differentiating cognitively distinct processes that might all be called planning. Stacking items to reach a goal might require different processes such as seriation of movement. Route planning might be more reliant on spatial navigation skills. And, anticipating the need to find and retrieve a tool that is needed to complete a task might rely on prospective memory capacities. Thus, the cognitive processes involved in different types of planning might not necessarily be identical, but they are all likely related, and all have some degree of future-orientation. As noted, there is some evidence that animals may plan on a relatively long time scale for future events, but there is a more extensive literature that reflects planning on a limited time scale in nonhuman animals.
One influential line of research into animal planning required animals to sequence visual stimuli in a particular order using a variation of the chained response task (e.g., D’Amato & Colombo 1988, 1989; Terrace, 1986). Some chimpanzees (Pan troglodytes) proved highly adept at seeing and then remembering a long sequence of needed responses when shown an array of to-be-sequenced stimuli for only brief intervals. These chimpanzees then completed the sequence based on their memory for the order that was needed for item selection (Inoue & Matsuzawa, 2007; see also Biro & Matsuzawa, 1999). Although not all chimpanzees performed this well on the task (Beran, Pate, Washburn, & Rumbaugh, 2004), all chimpanzees, as well as rhesus monkeys (Beran et al., 2004; Scarf & Colombo, 2009; Scarf, Danly, Morgan, Colombo, & Terrace, 2011; see also Washburn, 1992), capuchin monkeys (Cebus apella; Beran & Parrish, 2012) and pigeons (Columba livia; e.g., Scarf & Colombo, 2010), showed evidence of planning one or two moves ahead in similar sequencing tasks.
Another approach to examining choice behavior and potential planning in nonhuman animals involves the use of mazes and similar route-taking tasks (Fragaszy, Johnson-Pynn, Hirsh & Brakke, 2003; Fragaszy et al., 2009; Menzel & Menzel, 2007; Miyata & Fujita, 2008; Miyata, Itakura, & Fujita, 2009; Mushiake, Saito, Sakamoto, Sato & Tanji, 2001; Washburn, 1992). For example, Fragaszy et al. (2003) first trained chimpanzees and capuchin monkeys to use a joystick to navigate the alleys of computerized mazes consisting of up to five choice points to reach a rewarded goal area. The researchers then tested the animals on novel mazes including some that involved solutions that initially led away from the direction of the goal before reaching the end (and thus required animals to look beyond the next one or more turns before getting too far into the maze). Both species solved the mazes at rates above chance performance. However, capuchin monkeys erred more often than chimpanzees, especially when the correct alley led away from the goal. Fragaszy et al. (2009) extended this research by training capuchins and chimpanzees to perform a long series of mazes either in a random order or in an order of ascending solution difficulty (with regard to the number of choice points as well as turns that led away from the direction of the goal). Chimpanzees again outperformed capuchins and were unaffected by the training condition, whereas capuchins appeared to benefit from learning the mazes in ascending order. Most recently, Pan et al. (2011) further investigated the influence of task experience on maze performance in capuchins by having monkeys perform the same series of mazes as in Fragaszy et al. (2009) until they mastered them. These monkeys subsequently exhibited improved performance in novel transfer mazes, particularly those that involved solutions with detours initially leading away from the direction of the goal.
Another approach to assessing maze performance was through presentation of finger mazes to two chimpanzees (Iversen & Matsuzawa, 2001). Subjects had to move an onscreen “ball” through a visual maze presented on the screen. Both chimpanzees learned to do this with finger movements first by learning to move the ball around obstacles onscreen and then with training mazes. Their successful performances with the training mazes then generalized to new mazes, although not immediately. They also learned to approximate the optimal response path to the goal on many of these mazes. The one limitation in their flexible and proficient performance of the mazes came when a shorter path in the direction of the goal also had an obstacle that prevented the ball from reaching the goal. On those trials, the chimpanzees were equally likely to take that (impossible) path as they were to take the longer but possible path to the goal. Although this experiment was not specifically about planning abilities in chimpanzees, the success of chimpanzees with these mazes highlighted that such an approach could be used to assess planning skills.
Using a somewhat related approach, Tecwyn, Thorpe, and Chappell (2013) examined planning behavior in a manual maze task by apes. They devised a “paddle-box” task for use with bonobos (Pan paniscus) and orangutans (Pongo pygmaeus) that consisted of levels of short platforms (i.e., paddles) that had to be manipulated in order for a food item to pass from the top level to the bottom level of the maze where the animal could access the food. The researchers tested the apes in two experiments in which the lower paddles sometimes had to be arranged before manipulating the top paddle holding the food item in order to successfully guide the food from top to bottom. Apes only succeeded when they did not have to arrange lower paddles in advance, suggesting that they could not plan multiple steps in advance in this task (possibly because they could not inhibit a prepotent response to the food item and its initial location). Tecwyn, Thorpe, & Chappell (2014) extended this research to 4- to 10-year-old human children (Homo sapiens) and devised new measures to assess whether reducing the inhibitory control demands of the task would allow for greater planning ability. One measure involved requiring children to pause for a few seconds before performing the task, and the other measure replaced the food reward with a token representing the reward. Neither method influenced the extent to which children showed advanced levels of planning. These results could be interpreted to show that failed inhibition did not prevent children from succeeding in this planning task, or possibly that these manipulations were not effective.
Völter and Call (2014a) conducted a similar study to Tecwyn et al. (2013, 2014) with bonobos, chimpanzees, gorillas (Gorilla gorilla), orangutans, and 4- to 5-year-old human children in which the participants manipulated a reward item to the bottom of a vertical maze by sliding the item with a finger into gaps within the vertical levels. Different configurations of the maze required subjects to plan their movements up to two steps in advance in order to solve the puzzle, and researchers found that younger apes and older children successfully planned movements two steps in advance, while younger children planned one movement in advance, and older chimpanzees did not seem to plan their moves at all in this task. Apes’ performance was largely influenced by number of changes in direction (a potential measure of response inhibition, as suggested by Tecwyn et al., 2013), whereas children’s performance was more influenced by the number of sub-goals of the task (a potential measure of attention). Thus, there appear to be some differences in how human children, apes, and monkeys approach different kinds of maze tasks with regard to planning routes through those mazes, exhibiting the inhibition needed when reversals of direction are needed, and in the ability to anticipate “traps” that might occur later in a maze if one travels in a certain direction. All of these characteristics of mazes make them a good choice for assessing short time-scale planning abilities across species.
To provide a related but novel assessment of planning abilities across species, we designed a computerized maze task that operated visually much like the physical mazes used by Völter and Call (2014a, b) and Tecwyn et al. (2013, 2014). Our mazes were completed by using a joystick to move a cursor leftward or rightward onscreen until the cursor reached gaps at each level of the maze and “fell” to the next level. To successfully complete a trial, participants were required to move the cursor into contact with a goal location somewhere at the bottom of the screen, while avoiding traps and gaps that instead dropped the cursor onto the maze bottom without hitting the goal. We presented 100 unique mazes to an ape species (chimpanzees), an Old World monkey species (rhesus monkeys, Macaca mulatta), a New World monkey species (capuchin monkeys), and human children to provide a broad phylogenetic assessment of planning abilities in the order Primates.
Our mazes were designed so that participants had to make one, two, or three choices within the maze that potentially could have led to irreversible errors at those choice points. Additionally, each maze varied in a more conceptual way with regard to its degree of difficulty. Some mazes, independent of the number of objective choice points, could be completed by simply moving the cursor always in the direction of the goal. These were considered the easiest mazes. Other mazes, however, required a reversal of direction at one of the choice points. These reversals required a degree of manual inhibition and control so that after falling from one level to the next, the participant had to reverse the cursor direction or else an error would be made on that level. These mazes were considered to be challenging for any individual or species that struggled with motor control or behavioral inhibition with regard to maintaining a course of action when change was needed. Other mazes required a movement away from the goal so as to eventually reach it, whereas continued movement always toward the goal guaranteed an error. These trials challenged individuals (and species) to anticipate that moving toward the goal would lead to an error, whereas looking instead at the later effects of each movement could lead to recognition that the correct route required moving away from the goal. And, in the most difficult trials, a reversal and a movement away from the goal were required within the same maze.
We expected that an individual (or species) that showed planning behavior in terms of determining the correct route through the maze would perform equally well in all of these trial types, whereas difficulty with movements away from the goal in order to reach it or reversals in direction would reflect less planning ability and less inhibitory control in performing this task, respectively. Therefore, we predicted that chimpanzees would outperform the monkey species given past research showing greater planning abilities in these animals. We also predicted that chimpanzees might approach the performance levels of children overall, although we expected that older children would be more proficient with all maze types relative to younger children. Finally, we predicted that mazes requiring movements away from the end goal or a change of direction would prove especially difficult for the monkey species based on previous research (e.g., Fragaszy et al., 2003). Such changes might also prove difficult – although to a lesser degree – for chimpanzees, given past research showing that such movements sometimes disrupted performance in other maze tasks (Iversen & Matsuzawa, 2001; Völter & Call, 2014a).
There are multiple reasons to expect that species differences might be present in this kind of task. Fragaszy et al. (2009) outlined one ecological perspective for such differences. They proposed that chimpanzee ecology allowed for greater sustained attention to small parts of the visual field versus the need for much more vigilant scanning of all areas within view for primates such as capuchin monkeys that must be on the lookout for predators. Solving mazes would require more focused attention to a small visual stimulus, and thus even captive chimpanzees would have an advantage because of this predilection to sustained attention.
A second possibility is that chimpanzees benefit from greater general executive functioning abilities because of their larger brain size, and thus can show the inhibition and planning necessary to perform better on mazes. Brain size has been related to general cognitive ability in a variety of tasks (Deaner, Isler, Burkart, & van Schaik, 2007; Rumbaugh & Pate, 1984), and chimpanzees (and other apes) have the largest brains among primates. From this perspective, chimpanzees should exceed all monkeys in their performance on this maze task. From a more ecological perspective (that also has been linked to the selection for larger brain size in apes), it may be that the fission-fusion dynamics of chimpanzee social groups, that involve splitting and merging of members often and keeping track of many social interactions, requires a higher degree of inhibition in a rapidly changing social environment relative to the more stable and hierarchical environments of monkeys (see Amici, Aureli, & Call, 2008). This greater inhibition might also serve to facilitate better performance in maze-like tasks where acting in a prepotent manner without careful monitoring of the environment leads to trouble.
Fragaszy et al. (2009) suggested that it would be informative to observe performance in maze tasks by a primate species that differed in its vigilance (or distractibility) from both chimpanzees and capuchin monkeys. Rhesus monkeys would be one potential species for such a comparison. They have smaller brains than chimpanzees but larger brains than capuchin monkeys (e.g., Rilling & Insel, 1999). They also sometimes show greater executive functioning abilities in areas such as uncertainty monitoring compared to capuchin monkeys (e.g., Beran, Perdue, & Smith, 2014; Beran & Smith, 2011; Beran, Smith, Coutinho, Couchman, & Boomer, 2009). And, they perform similarly to chimpanzees on other metacognition tasks (e.g., Call & Carpenter, 2001; Hampton, Zivin, & Murray, 2004) but capuchin monkeys typically fail to match such performances (Basile, Hampton, Suomi, & Murray, 2009). However, in other tasks such as prospective memory and monitoring tasks, they perform equivalently to capuchin monkeys (e.g., Evans & Beran, 2012). Thus, it would be informative to compare rhesus monkeys to capuchin monkeys and chimpanzees in an effort to indirectly examine what ecological and biological factors may impact maze task performance.
Experiment 1 – Nonhuman Primates
Participants
We tested seven adult male rhesus monkeys (Chewie: age 13; Gale: age 29; Han: age 10; Hank: age 29; Luke: age 13; Murph: age 19; and Obi: age 9). Rhesus monkeys were housed individually at the Georgia State University Language Research Center (LRC), but had constant visual and auditory access to other nearby monkeys, in addition to a 24-hour period with direct access to a compatible social partner once per week. All rhesus monkeys had 24-hour access to water and were fed manufactured chow, fruits and vegetables daily between 1700 and 1800 hours.
We tested eight capuchin monkeys including four males (Griffin: age 15; Liam: age 9; Logan: age 7; and Nkima: age 5) and four females (Gambit: age 16; Lily: age 15; Nala: age 10; and Wren: age 10). Capuchin monkeys were group housed at the LRC but voluntarily shifted into individual enclosures for testing. All capuchins had 24-hour access to water and were fed manufactured chow following test sessions, as well as various fruits and vegetables between 1600 and 1700 hours.
We tested 4 chimpanzees, including two males (Mercury: age 27; and Sherman: age 40) and two females (Lana: age 43; and Panzee: age 28). All chimpanzees were housed together in the same building at the LRC and spent time together in social groups daily, but they were tested separately. Chimpanzees received a full diet of fruit, vegetables, and primate chow at multiple times each day and had ad libitum access to water.
All primates had participated previously in multiple psychological experiments involving the computerized test system used in this study (e.g., Beran, 2006, 2008; Beran, Evans, Klein, & Einstein, 2012; Beran & Parrish, 2013; Beran & Smith, 2011; Beran & Washburn, 2002; Evans & Beran, 2012, 2014; Evans, Perdue, Parrish, & Beran, 2014; Klein, Evans, Schultz, & Beran, 2013). All chimpanzees had some experience in working on computerized mazes that looked different from the present mazes but that still involved moving to a goal on a computer screen (Fragaszy et al., 2003, 2009). Some rhesus monkeys also had performed different kinds of spatial detour tasks that approximated maze-like tasks (e.g., Menzel & Menzel, 2007). These capuchin monkeys had not performed any type of computerized maze before this study. None of the animals, however, had ever performed a computerized variation of a vertical maze where the cursor appeared to fall down the levels of the maze.
Apparatus and Materials
The nonhuman primates were tested using the Language Research Center’s Computerized Test System (LRC-CTS) comprising a personal computer, digital joystick, color monitor, and pellet dispenser (Evans, Beran, Chan, Klein, & Menzel, 2008; Richardson, Washburn, Hopkins, Savage-Rumbaugh, & Rumbaugh, 1990). Primates manipulated the joystick with their hands to produce isomorphic movements of a small cursor on the computer screen. Contacting stimuli with the cursor sometimes resulted in the delivery of a food reward. Monkeys were rewarded with 45-mg (capuchins) or 94-mg (rhesus) banana-flavored chow pellets (Bio-Serv, Frenchtown, NJ) via a pellet dispenser interfaced to the computer through a digital I/O board (PDISO8A; Keithley Instruments, Cleveland, OH). Chimpanzees were rewarded with fruit pieces delivered by hand by an experimenter who otherwise could not see the computer screen and responded only to the feedback tones produced by the computer. The task program was written in source code using Visual Basic 6.0.
Design and Procedure
Training
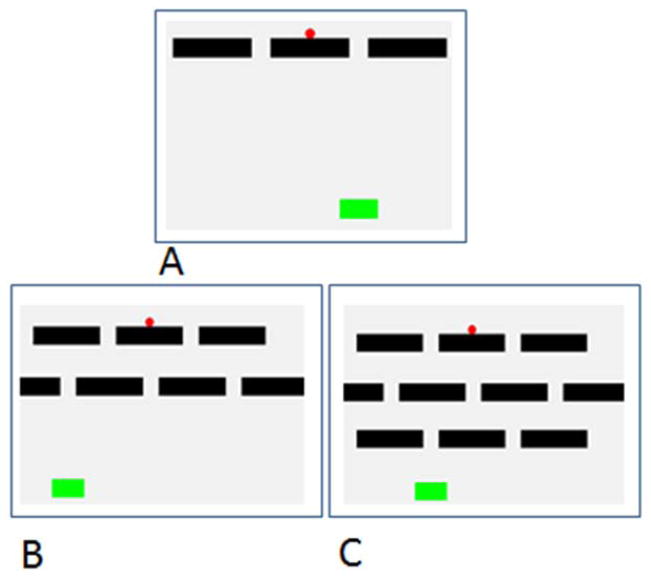
Training with the computerized maze program progressed through three phases. In Phase 1 (Figure 1a), the maze consisted of a single level at the top of the computer screen, with that level consisting of three bars, with gaps between the two end bars and the screen edge, as well as between the middle bar and each end bar. The cursor randomly appeared on one of the three bars (as if resting on top of it), and the goal location (a green rectangle) appeared at the bottom of the screen in one of seven possible locations, although that location on a given trial had to be in one of the possible locations to which the cursor could fall. The subject moved the joystick to move the cursor left or right until it reached a gap at which point it fell downward automatically into contact with the goal or into empty space. Contact with the goal led to a melodic chime and food reward whereas missing the goal led to a buzz tone and a timeout period (8 seconds for chimpanzees, 20 seconds for monkeys) before presentation of the next trial. Different timeout periods were used with different species because these were the standard periods used with each species in other forms of computerized testing. When a subject was correct on at least 12 of the most recent 15 trials, they progressed to the next phase.
Figure 1.
Training mazes from the study. The small circle on the top row is the cursor, and the solitary rectangle at the bottom of each image is the goal location. A. Phase 1. B. Phase 2. C. Phase 3.
In Phase 2, there were two levels presented, and so now the cursor fell from a gap in the top level of the maze to the next level of the maze, and then after being moved to another gap, fell to the bottom of the screen and either landed on the goal or did not (Figure 1b). Thus, subjects had to make two movements of the cursor to get it through the maze, whereas they had to make one movement in the previous phase. The training criterion was the same, and in Phase 3 a third level was added (Figure 1c). This trained the participants about the need to make multiple movements through the maze, and also how to reverse the direction of the cursor.
When a subject met criterion on Phase 3, the subject then moved to Phase 4 in which vertical walls were introduced into the maze. These blocked lateral movement of the cursor and forced a change in direction, and these trials were presented to teach the subjects about how walls could block progress. Each participant was given extensive experience with these mazes prior to moving to the formal test phase, and nearly all participants became quite proficient with this phase of training (see Results).
Test Phase
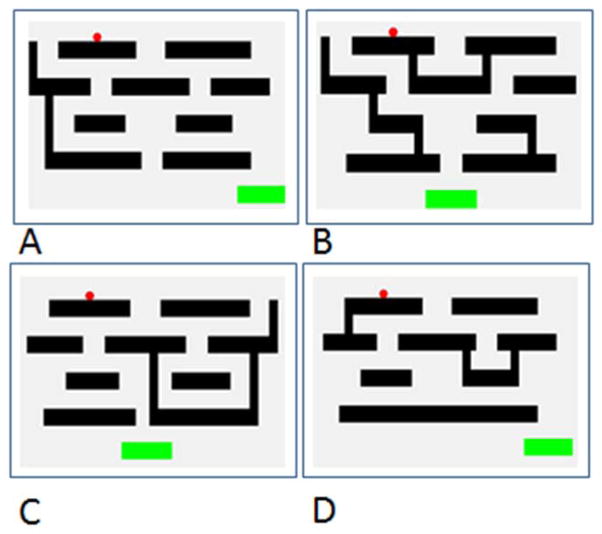
Each maze now consisted of four levels (see Figure 2). As in training, the cursor began on each trial at the top level, and the remaining screen area consisted of parallel horizontal lines that contained gaps through which the cursor could pass to move from higher to lower levels of the maze. Some of these gaps brought the cursor closer to the goal, whereas others brought the cursor farther away from the goal. Gaps occasionally even dropped into a trap (a bar section blocked by a vertical wall on each side) that prevented the participant from completing the trial (e.g. see Figure 2b). Mazes were randomly designed, and 100 unique mazes were generated. Each trial therefore varied in the start location of the cursor, the goal location, the locations of the gaps, traps, and walls, and the number of movements required.
Figure 2.
Example test mazes. A. Direct Trial. B. Away Trial. C. Reversal Trial D. Reversal + Away Trial
Each maze could be objectively defined in terms of the number of critical choices needed, and this number ranged from one to three choices. Critical choices were those for which an incorrect choice led to a guaranteed (and irreversible) error, such as missing the goal when the cursor finally reached the bottom of the maze, or falling into a trap. When participants fell into a trap, the cursor remained active but could not go anywhere, and this continued until a set time interval elapsed for the trial, after which the program provided a buzz tone, cleared the screen, and began the timeout period. This interval was 30 seconds for the chimpanzees, 60 seconds for the rhesus monkeys, and 90 seconds for the capuchin monkeys. These values differed because of unique species’ responses to such delays, and because we wanted an interval that would make falling into the traps sufficiently aversive to try to get subjects to avoid those traps and to complete the mazes correctly. Chimpanzees were most likely to discontinue working the task altogether when delays were longer than 30 seconds, and capuchin monkeys were most likely to keep working on trials even after experiencing the 90 second interval.
Each maze also was classified as requiring a move away from the goal at one of these critical choice points (“Away Trials”) or not requiring such a move (“Direct Trials”) In Away Trials, the subject had to move the cursor in the opposite direction of the goal (e.g., move the cursor to the left even if the goal was located to the right of the cursor). Thus, a subject had to see that continuing toward the goal would lead to an error on that level or a future level of the maze (Figure 2b). Each critical choice point in a maze also could require (or not) a reversal of cursor movement from the direction in which it had been heading (“Reversal Trials”; Figure 2c). Reversal Trials did not necessarily require moving away from the goal, but did require stopping movement in one direction that was ongoing at a choice point and moving in the opposite direction. These reversals and moves reflected motor control (and inhibition) of cursor movement and reflected the difficulty of negotiating the maze from the perspective of controlling cursor movement and reversing ongoing activity. Some mazes required a reversal and a move away from the goal, and these were considered the most difficult mazes in the series (“Reversal + Away Trials”; Figure 2d). Overall, each subject completed 200 trials (two trials with each of the 100 unique mazes, with all 100 unique mazes being completed in the first block of trials and then being repeated in the second block). Within these 200 trials, 48 trials were Away trials, 50 were Reversal trials, and 38 were Reversal + Away trials. The remaining 64 trials were Direct trials. These maze types were randomly presented from trial to trial.
In all phases of the experiment, chimpanzees typically worked on the task for 30 to 60 minutes per session, and monkeys worked on the task for two to four hour blocks of time. Chimpanzees continuously worked with an experimenter present, and so they tended to stay on task throughout the session although they occasionally took short breaks to drink water or to watch other areas of the building. Monkeys worked on the automated system and rested as they chose throughout the longer session. Thus, sessions were self-paced to a large degree by the motivation of the animal to engage the task. This meant that different numbers of trials were generated each session by each animal because of this freedom to work and rest as they chose.
Results
Training
Table 1 presents the number of sessions, total trials, trials at the final training level, and percentage of trials correct at the final level. All subjects except for one capuchin monkey (Wren) reached the final training phase, although performance at that level varied greatly.
Table 1.
Training performance (number of sessions and number of trials at each level) for each subject in Experiment 1
| # Sessions | Phase 1 trials | Phase 2 trials | Phase 3 trials | Phase 4 Trials | % Correct in Phase 4 | |
|---|---|---|---|---|---|---|
| Chimpanzees | ||||||
| Lana | 4 | 108 | 19 | 13 | 174 | 74.1% |
| Mercury | 2 | 65 | 20 | 13 | 122 | 85.2% |
| Panzee | 4 | 16 | 15 | 13 | 217 | 88.9% |
| Sherman | 4 | 16 | 15 | 15 | 234 | 76.5% |
| Rhesus monkeys | ||||||
| Chewie | 8 | 237 | 25 | 50 | 168 | 58.9% |
| Gale | 8 | 136 | 77 | 18 | 275 | 68.0% |
| Han | 4 | 412 | 34 | 15 | 769 | 77.5% |
| Hank | 9 | 311 | 135 | 180 | 329 | 64.1% |
| Luke | 5 | 45 | 39 | 47 | 221 | 71.5% |
| Murph | 6 | 18 | 14 | 17 | 620 | 73.2% |
| Obi | 5 | 224 | 65 | 19 | 755 | 81.6% |
| Capuchin monkeys | ||||||
| Griffin | 7 | 860 | 49 | 115 | 250 | 57.6% |
| Liam | 7 | 358 | 15 | 14 | 540 | 54.6% |
| Lily | 9 | 568 | 131 | 45 | 278 | 60.8% |
| Logan | 6 | 404 | 123 | 26 | 710 | 75.8% |
| Nala | 4 | 180 | 40 | 24 | 957 | 77.7% |
| Nkima | 4 | 43 | 34 | 48 | 1206 | 74.7% |
| Widget | 6 | 315 | 72 | 43 | 216 | 38.0% |
| Wren | 5 | 691 | 531 | 52 | NA* | NA* |
Note: Wren did not reach criterion at Level 3 when her training ended.
Test Phase
The chimpanzees each required two sessions to complete the 200 mazes. The rhesus monkeys required 3 to 11 sessions (Chewie – 3; Gale – 8; Han – 4; Hank – 6; Luke – 11; Murph – 4; Obi – 5). The capuchin monkeys required 1 to 6 sessions (Griffin – 5; Liam – 3; Lily – 6; Logan – 1; Nala – 2; Nkima – 1; Widget – 5; Wren – 5).
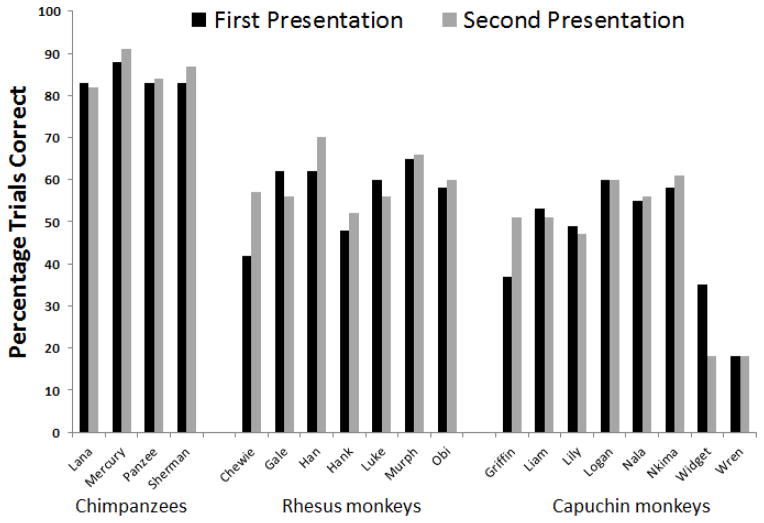
The first thing that we assessed was whether performance differed between the first and second presentation of each specific maze, to determine if there were any learning effects (Figure 3). None of the chimpanzees showed any improvement on the second presentation of the mazes compared to the first presentation, all Χ2 (1, N = 200) < 1.0, p > .10. Only one rhesus monkey (Chewie) showed a significant improvement, Χ2 (1, N = 200) = 4.5, p < .05, whereas all other rhesus monkeys did not, Χ2 (1, N = 200) < 1.50, p > .10. Only one capuchin monkey (Griffin) showed a significant improvement, Χ2 (1, N = 200) = 3.98, p < .05. One capuchin monkey (Widget) performed significantly worse on the second presentation of the mazes, Χ2 (1, N = 200) = 7.42, p < .05. All other capuchin monkeys showed no difference in performance, Χ2 (1, N = 200) < 1.00, p > .10. Thus, experience with a particular maze generally did not improve performance when seeing that same maze for a second time in the current study, and so we collapsed across presentation block for all remaining analyses.
Figure 3.
Comparison of performance for each nonhuman primate for the first and second presentation of all mazes.
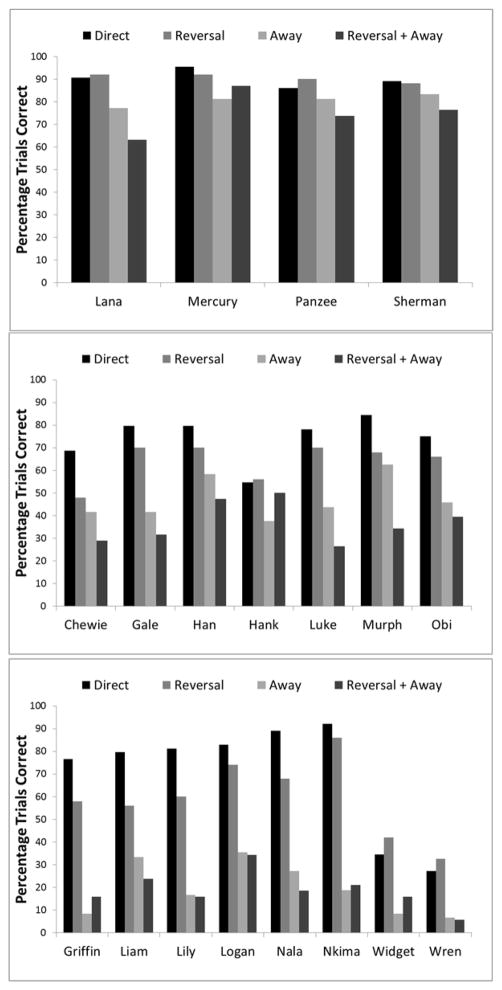
To assess the degree of trial difficulty and its impact on the performance of species and individuals, we separated all mazes into one of four categories as outlined in the Methods section (Direct Trials, Reversal Trials, Away Trials, and Reversal + Away trials). Here, the number of choice points was not relevant because the trials were defined on the basis of their difficulty in terms of requiring (or not) moves away from the goal and reversals of ongoing direction. This decision was made because it was found that an increase in critical choices did not necessarily warrant an increase in difficulty. It would be possible for a maze to have three critical choice points, but the correct choices at each point to be in the same direction toward the end goal (see Figure 2A) which would subsequently be relatively easy for a subject focused only on moving in the direction of the end goal. To analyze the effect of these trial types on performance, we conducted a repeated measures ANOVA with maze type as a within-subjects factor and species as a between-subjects factor. There was a significant main effect of species, F(1, 16) = 631.2, p < .001, ηp2 = .75, and a main effect of maze type, F(3, 48) = 62.81, p < .001, ηp2 = .80. However, these main effects were qualified by a significant interaction between species and maze type, F(6, 48) = 7.66, p < .001, ηp2 = .49.
To better assess the differences in performance across maze types, we conducted paired t-tests for all four maze types for each species. To control for family-wise error rate given the repeated tests, we applied the Bonferroni correction and set alpha at .0083. The results of these tests are shown in Table 2. Individual results for each member of each species are shown in Figure 4 to provide information about how each animal performed. In general, all members of the same species appeared to perform similarly, with the exception that two capuchin monkeys (Widget and Wren) who were very poor performers overall.
Table 2.
Comparison of performance in each pairing of maze conditions for each species in Experiment 1 with Alpha = .0083 (Bonferonni correction)
| Chimpanzees | Reversal | Away | Reversal + Away |
|---|---|---|---|
| Direct | t(3) = −.67, p = .878 | t(3) = 3.81, p = .032 | t(3) = 3.64, p = .036 |
| Reversal | t(3) = 4.58, p = .020 | t(3) = 3.10, p = .053 | |
| Away | t(3) = 1.40, p = .255 | ||
| Capuchin monkeys | Reversal | Away | Reversal + Away |
| Direct | t(7) = 2.47, p = .043 | t(7) = 7.37, p < .001 | t(7) = 7.00, p = .001 |
| Reversal | t(7) = 8.10, p < .001 | t(7) = 9.00, p < .001 | |
| Away | t(7) = .23, p = .83 | ||
| Rhesus monkeys | Reversal | Away | Reversal + Away |
| Direct | t(6) = 3.94, p = .008 | t(6) = 9.56, p < .001 | t(6) = 6.09, p = .001 |
| Reversal | t(6) = 4.83, p = .003 | t(6) = 5.62, p = .001 | |
| Away | t(6) = 2.24, p = .066 |
Figure 4.
Comparison of the four maze types for each nonhuman primate species. Chimpanzees are shown in the top panel, rhesus monkeys are shown in the middle panel, and capuchin monkeys are shown in the bottom panel.
Discussion
Chimpanzees clearly succeeded with all types of maze trials in this experiment. They were nearly as proficient when they had to move away from the goal and when they had to reverse directions, as when they did not need to do these things. Statistically, performance was equivalent, although it is important to note that the sample size was small for chimpanzees, and an examination of the data for individual chimpanzees in Figure 4 does show that performance sometimes did suffer for the more difficult trial types. Overall, though, the data suggest that these four chimpanzees examined the mazes, noticed the potential error-causing areas within the maze, and completed the mazes even when they have to make multiple decisions within the maze. These data complement other tasks given to chimpanzees that also show they are capable of some degree of planning (e.g., Fragaszy et al., 2003, 2009; Völter & Call, 2014a).
The monkeys were less proficient. Both species proved fairly capable when they could move directly to the goal location and did not have to reverse cursor direction or move away from the goal. Both species had particular difficulty when the mazes required them to move the cursor away from the goal location (either independent of – or in combination with – having to reverse directions), and this suggested rather limited abilities to plan movements through this form of maze, perhaps as a result of behavioral inhibition problems or difficulty in seeing future trouble spots within the maze. We return to these issues in the General Discussion, where we compare the overall performances of all nonhuman primate species to the performances of human children tested in Experiment 2.
Experiment 2 - Children
In Experiment 2, we gave children the same 100 unique mazes that the nonhuman primates completed in the test phase of Experiment 1. This allowed us to directly compare children’s performance against that of the nonhuman primates, and specifically to see if the same factors predicted success or failure for children as they had for the nonhuman primates. These data also were valuable in comparison to previous work using computerized or manual mazes with children in tasks that could be compared to performances from nonhuman species (e.g., Miyata et al., 2009; Tecwyn et al., 2013, 2014).
Participants
We tested 27 children ranging in age from 28 months to 66 months (13 females and 14 males). All children were tested at a local preschool near Atlanta, Georgia. Children were tested during normal school hours. Experimenters brought pairs of children from their classrooms to the testing room where they each worked on the task on a separate computer and with the aid of a separate experimenter. Parents of the children consented to their participation in the study, and children chose if they wanted to work with the experimenters, and when they wanted to stop working during each session.
Materials
Children performed the task on laptop computers with digital joysticks using the same computer program as the nonhuman primates. However, rather than food rewards, children received visual and auditory feedback following correct/incorrect trials (i.e., happy/sad faces and melodic/buzz tones). Additionally, children received a sticker and/or small toy of their choice at the end of each test session as a reward for participating, regardless of how many trials they completed correctly or incorrectly.
Design and Procedure
The experimenter sat next to the child and reminded them at the outset of each test session that they needed to move the cursor down the maze and try to land on the green rectangle. Otherwise, the experimenter only provided verbal praise for the child’s efforts, and also sometimes interacted with the child to provide short breaks from working. All children completed multiple sessions in the experiment, as they typically performed somewhere between 10 and 30 mazes in a session.
One child (29 months of age) showed great difficulty manipulating the joystick while looking at the computer screen, and so that child was removed from the study. The data from that child were not included in the analyses. Twenty children completed all 100 mazes, one time only, but six children did not finish the experiment because they left the school during the study. Four of those children (ages 44, 62, 64, and 66 months) completed at least 50 mazes, and the remaining two children (28 and 60 months) completed 36 and 32 mazes. We included the data from those children in all analyses. The mean number of test sessions completed for this group of children was 5.5, with a range of 2 to 8 sessions.
Results
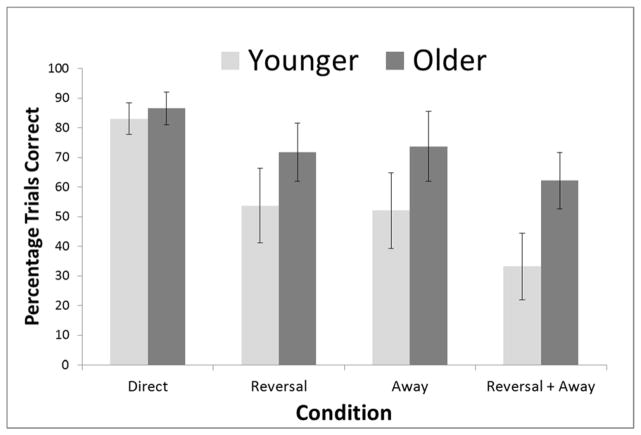
To assess the degree of trial difficulty and its impact on the performance of individuals and age groups, we again separated all mazes into one of four categories as outlined in the Method section (Direct Trials, Reversal Trials, Away Trials, and Reversal + Away trials). To analyze the effect of these trials types on performance, we conducted a repeated measures ANOVA with maze type as a within-subjects factor and age group as a between-subjects factor. For age groups, there was a clear point for splitting the children into older and younger age groups. Of the 26 children in the analysis, 13 were 48 months or younger at the start of the experiments, and 13 were 54 months or older. The results for these two age groups for each condition are shown in Figure 5. There was a significant main effect of maze type, F(3, 72) = 37.74, p < .001, ηp2 = .61, and a main effect of age group, F(1, 24) = 9.12, p = .006, ηp2 = .27, with older children outperforming younger children. These effects were qualified by a significant interaction of age group and maze type, F(3, 72) = 4.65, p = .005, ηp2 = .16.
Figure 5.
Comparison of the four maze types for children separated into a Young age group (48 months and younger) and an Old age group (54 months or older).
To investigate performance more closely, we conducted paired t-tests for all four maze types for children in each age group. Given the repeated tests, we applied the Bonferroni correction and set alpha at .0083. Younger children performed significantly better on Direct trials than on Away Trials, t(12) = 4.85, p < .001, and significantly better on Direct trials than on Reversal + Away Trials, t(12) = 10.51, p < .001. They also performed significantly better on Away Trials than on Reversal + Away Trials, t(12) = 4.53, p = .001. With the corrected alpha level, there was no significant difference between Direct trials and Reversal Trials, t(12) = 2.87, p = .014. There also was no significant difference between Reversal Trials and Reversal + Away Trials, t(12) = 2.85, p = .015, and there was no significant difference between Reversal Trials and Away Trials, t(12) = 1.04, p = .32.
Older children performed significantly better on Direct trials than on Reversal Trials, t(12) = 4.38, p = .001 and better on Direct trials than on Reversal + Away Trials, t(12) = 4.68, p = .001. They also performed significantly better on Away Trials than on Reversal + Away Trials, t(12) = 4.30, p = .001. There was not a significant difference between Direct trials and Away Trials, t(12) = 1.96, p < .073, between Reversal Trials and Reversal + Away Trials, t(12) = .17, p = .86, or between Reversal Trials and Away Trials, t(12) = 1.00, p = .34.
Discussion
Across the age range of 28 months to 66 months, children were successful to varying degrees in learning to perform these computerized mazes. Our opportunity to repeatedly test the children so that many of them could complete as many as 100 of these mazes allowed us to present a variety of different maze types as in Experiment 1. The results in some ways paralleled those from the two monkey species in Experiment 1, but in other ways more closely matched the performance of the chimpanzees. We discuss these comparisons in the General Discussion in more detail.
It is important to note that there were some methodological differences that require caution in making too direct a comparison between the performance of children and that of the nonhuman primates. The children were verbally instructed as to the goal of the task whereas the nonhuman primates had to learn this, and did so through hundreds of trials of exposure to the training phases that we designed. The nonhuman primates also completed two trials each of the 100 unique mazes instead of just one trial. Although there was not a difference between first and second exposure to those mazes, this still meant there was a difference in experience across the species. And, children did not work for food rewards whereas nonhuman primates did, and this could have influenced their motivation to perform that task.
Our results matched previous reports that showed an effect of age on performance in other variations of maze tasks (e.g., Miyata et al., 2009; Tecwyn et al., 2014). We had predicted better performance for older children on this task as well, and that was the case for all conditions except the easiest one. Thus, such maze tasks in computerized format, including the need for responding through joystick use, can be a useful tool in assessing spatial abilities and planning abilities in children.
General Discussion
Four species, including human children, a great ape (chimpanzees), an Old World primate (rhesus monkeys) and a New World primate (capuchin monkeys) completed the same computerized maze task in an effort to understand the relative competencies of these species, and the degree to which certain aspects of maze presentation might disrupt performance. The results were illuminating, specifically with regard to the role of inhibition and anticipation of trouble spots within the maze that necessitated moving away from the goal in order to reach it. These factors contributed differently to the performance of each species although the overall trends among the four species were quite similar.
Among the nonhuman primates, the chimpanzees clearly performed the best. In fact, of all species, the chimpanzees alone showed no significant differences among the four qualitative kinds of mazes in terms of what behavioral responses were required in those mazes (i.e., reversals, moves away from the goal, or both). However, it is important to note that only four chimpanzees were tested, and these were highly experienced individuals in terms of cognitive testing on computerized tasks, including some experience with computerized planning tasks. And, a visual inspection of Figure 4 indicates that even the chimpanzees showed apparent performance decrements for reversals and moves away from the goal (especially Lana). But, overall, the chimpanzees seemed to show clear evidence that they were investigating routes through the maze, because they anticipated the need to move away from the goal sometimes, and they also showed the motoric inhibition needed to execute the reversals. These chimpanzees showed better planning abilities than the monkeys, and in particular may have excelled with regard to the inhibitory requirements of the task, even relative to other apes given maze tasks (e.g., Tecwyn et al., 2013). For example, Iversen and Matsuzawa (2001) found that chimpanzees preferred short paths toward a goal over long paths to the goal even when the short path was blocked. And, Völter and Call (2014a) noted that chimpanzees sometimes struggled more when maze tasks involved direction reversals than when they did not. The chimpanzees that were tested here did not seem to struggle as much with these task demands, but this could be the result of our different kind of planning maze rather than being something different about these chimpanzees. Despite some of these performance differences, which may be the result of methodological variations, our chimpanzees’ results overall complement those found in other kinds of tasks with chimpanzees with regard to showing that chimpanzees do show some degrees of planning behavior (e.g., Beran et al., 2004; Biro & Matsuzawa, 1999; Fragaszy et al., 2003; Iversen & Matsuzawa, 2001; Völter & Call, 2014a).
The monkey species showed two consistent patterns. First, the need to reverse the cursor direction at some point came at a performance cost relative to trials in which the cursor could always move directly to the goal. Second, the need to move away from the goal added another (seemingly larger) performance cost, and for most monkeys, trials with both requirements were extremely difficult and were performed at the lowest levels. This indicates that monkeys showed both inhibitory difficulties and difficulty anticipating trouble spots that require moving away from the goal. A comparison of performance on the first and second presentation of each maze indicated that performance did not improve with experience with a specific maze, and so this may be a real limitation in monkey planning abilities, although it remains to be determined if this limitation would hold for other testing paradigms or after some attempt at remediation for these inhibitory difficulties (e.g., increased training and experience; see Fragaszy et al., 2009; Pan et al., 2011).
There are several potential explanations to account for the observed differences in monkey and ape performance on these computerized mazes and previously reported differences (e.g., Fragaszy et al., 2003, 2009; Pan et al., 2011). The chimpanzees tested in our experiment have participated in a number of automated maze experiments that vary in their degree of difficulty and inhibitory requirements (Fragaszy et al., 2003; Menzel & Menzel, 2007). These previous experiments provided the chimpanzees with more opportunities to learn about the nature of navigating 2-dimensional mazes with a joystick and potential strategies for doing so successfully (e.g., looking ahead to the end goal) in comparison to the monkey species that we tested. Although this experience may have aided the chimpanzees’ performances, naïve chimpanzees in other studies also have demonstrated higher performance levels in comparison to capuchin monkeys, and subsequently benefitted more from practice in maze tasks than did monkeys (Fragaszy et al., 2009).
The chimpanzees outperformed the monkey species because they were better at inhibiting movements towards the end goal if a maze required a change in direction or traveling away from the goal for a correct response. If this was the main difference in performance, it would suggest that the chimpanzees and monkeys might all perceive the correct routes through the mazes, but that the monkeys were unable to complete the necessary inhibitory behaviors to carry out the appropriate route. Alternatively, the monkeys may have failed to “plan ahead” prior to maze completion, instead only attending to one level at a time and losing focus on the whole maze. This greater susceptibility to distraction would lead to irreversible mistakes in mazes that required changes in direction or moves away from the end goal. Although the chimpanzees may have planned their route through the mazes more so than the monkeys, more research is needed to explore this hypothesis (e.g., eye-tracking data during automated maze completion). And, this difference does not necessarily hold across all tasks that assess planning abilities. For example, capuchin monkeys and rhesus monkeys performed similarly to chimpanzees on a sequential responding task that required sequencing visual stimuli in a learned order, suggesting that the planning abilities between great apes and monkeys may be more closely related in other kinds of tasks (Beran & Parrish, 2012; Beran et al., 2004).
There are also some biological and ecological aspects of these species that may have contributed to performance differences in the current planning task. These include differences in focused attention (versus vigilant scanning), differences in overall brain size, and differences in social systems (fission-fusion versus stable hierarchies). Fragaszy et al. (2009) proposed that wild capuchin monkeys, being smaller-bodied animals than chimpanzees, must engage in much more vigilant scanning of all areas within view to be on the lookout for predators, whereas chimpanzees can afford to focus greater sustained attention to small parts of the visual field without risk of predation. Rhesus monkeys are significantly larger than capuchin monkeys, but they are still occasionally preyed upon (Fooden, 2000), and therefore may also divide their attention between what is happening in front of them and what is happening around them. Thus, chimpanzees may have outperformed both monkey species in the current maze task because they were better able to focus their attention on the path of the cursor long enough to foresee upcoming pitfalls.
A second possibility is that chimpanzees outperformed the monkey species because they have larger brains and benefit from greater general executive functioning abilities, and thus can show the inhibition and prospection necessary to perform better on mazes. Greater executive capacities such as inhibition may have emerged in chimpanzees because of their greater need to track the splitting and merging of groups members that often occurs in fission-fusion social systems, relative to the more stable and hierarchical environments of monkeys (Amici et al., 2008). This greater inhibition, in particular, may have served to facilitate better performance in our maze-like task where acting in a prepotent manner without careful monitoring of the environment led to task failure.
In terms of absolute performance levels, children matched the performance of chimpanzees. Many of the children completed 80% or more of the mazes correctly, and some did so even for the mazes requiring reversals or moves away from the goal. Older children performed better than younger children, although both groups showed the same negative effects of the requirement for a reversal or a move away from the goal, although those two requirements produced about the same general degree of performance decrement. These two manipulations required a greater degree of behavioral inhibition and anticipation, respectively, than the Direct maze trials. As with the monkeys, there seems to be a clear relation between inhibition and performance on these kinds of mazes, but the question is whether the need to plan routes through the mazes disrupts the inhibitory skills, or whether the difficulties with inhibition produce the troubles in performance with the mazes. As noted by Tecwyn et al. (2014), testing children on other tasks requiring behavioral inhibition as well as mazes that require planning routes could be highly informative about the role of inhibition in planning, and also about how much having to plan can tax inhibitory processes and vice versa (McCormack & Atance, 2011). Our data echo this idea, and suggest that there is a clear link across numerous species in the general inhibitory abilities and planning abilities of individuals.
Ideally, one could measure the eye tracking behavior of children and nonhuman primates during this task, as has sometimes been done in other test paradigms (e.g., Scarf & Colombo, 2009; Scarf, Terrace, Colombo, & Magnuson, 2014), but we did not have the ability to do that in this experiment. Anecdotally, the children often made spontaneous verbal announcements about the mazes and their actions within the maze. Specifically, children sometimes noted that they saw a trouble spot further in the maze (e.g., “if I keep going that way, I will fall into the trap”), or they announced the general difficulty of the maze (e.g., “I have to go slow here so I do not fall off the edge and lose”). They also sometimes pre-traced their routes through the maze with a finger, or just by saying things such as “first I will go that way, but then I have to go the other way before turning back again.” These behaviors and verbal reports indicated attempts to plan successful routes through the maze, and undoubtedly contributed to better performance. Future research could more carefully control the use of such verbal or motoric anticipatory route planning, and perhaps use such behaviors as a means to facilitate inhibition and promote better performance on complicated mazes.
In summary, four species of primates, including human children, showed variable degrees of success with computerized mazes that required, in some cases, anticipation of future responses for correct maneuvering of the full maze, and also varying degrees of inhibition of movement for successful completion of the mazes. Children and chimpanzees outperformed monkey species, likely due to better inhibition and greater skill at recognizing the need to avoid future trouble spots before they were encountered. However, within each species (and across ages in children) there was variability in performance, suggesting that a number of other cognitive processes may influence planning processes and also may be influenced by the use of such processes. Clear candidates are various inhibitory processes, but at least for children there also may be some verbal processes such as talking aloud about the mazes prior to completing the mazes that affect performance. This study showed the viability of a “vertical” maze under joystick control as a comparative tool for assessing such processes, and for providing more insights into future oriented and motoric behaviors.
Acknowledgments
This research was supported by Grant HD060563 from the National Institutes of Health and Grant BCS-0924811 from the National Science Foundation. The authors thank the animal care staff at Georgia State University, the administrators and teachers at Suburban Nursery School and Pre-K in Atlanta, GA, and the parents of the children for their assistance and support for this research project.
Contributor Information
Michael J. Beran, Georgia State University
Audrey E. Parrish, Georgia State University
Sara E. Futch, Wofford College
Theodore A. Evans, Georgia State University
Bonnie M. Perdue, Agnes Scott College
References
- Amici F, Aureli F, Call J. Fission-fusion dynamics, behavioral flexibility, and inhibitory control in primates. Current Biology. 2008;18:1415–1419. doi: 10.1016/j.cub.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Babb S, Crystal J. Discrimination of what, when, and where: Implications for episodic-like memory in rats. Learning and Motivation. 2005;36:177–189. [Google Scholar]
- Basile BM, Hampton RR, Suomi SJ, Murray EA. An assessment of memory awareness in tufted capuchin monkeys (Cebus apella) Animal Cognition. 2009;12:169–180. doi: 10.1007/s10071-008-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ. Quantity perception by adult humans (Homo sapiens), chimpanzees (Pan troglodytes), and rhesus macaques (Macaca mulatta) as a function of stimulus organization. International Journal of Comparative Psychology. 2006;19:386–397. [Google Scholar]
- Beran MJ. Monkeys (Macaca mulatta and Cebus apella) track, enumerate, and compare multiple sets of moving items. Journal of Experimental Psychology: Animal Behavior Processes. 2008;34:63–74. doi: 10.1037/0097-7403.34.1.63. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Evans TA, Klein ED, Einstein GO. Rhesus monkeys (Macaca mulatta) and capuchin monkeys (Cebus apella) remember future responses in a computerized task. Journal of Experimental Psychology: Animal Behavior Processes. 2012;38:233–243. doi: 10.1037/a0027796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Parrish AE. Sequential responding and planning in capuchin monkeys (Cebus apella) Animal Cognition. 2012;15:1085–1094. doi: 10.1007/s10071-012-0532-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Parrish AE. Visual nesting of stimuli affects rhesus monkeys’ (Macaca mulatta) quantity judgments in a bisection task. Attention, Perception, & Psychophysics. 2013;75:1243–1251. doi: 10.3758/s13414-013-0474-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Pate JL, Washburn DA, Rumbaugh DM. Sequential responding and planning in chimpanzees (Pan troglodytes) and rhesus macaques (Macaca mulatta) Journal of Experimental Psychology: Animal Behavior Processes. 2004;30:203–212. doi: 10.1037/0097-7403.30.3.203. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Perdue BM, Bramlett JL, Menzel CR, Evans TA. Prospective memory in a language-trained chimpanzee (Pan troglodytes) Learning and Motivation. 2012;43:192–199. doi: 10.1016/j.lmot.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Perdue BM, Smith JD. What are my chances? Closing the gap in uncertainty monitoring between rhesus monkeys (Macaca mulatta) and capuchin monkeys (Cebus apella) Journal of Experimental Psychology: Animal Learning and Cognition. 2014;40:303–316. doi: 10.1037/xan0000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Smith JD. Information seeking by rhesus monkeys (Macaca mulatta) and capuchin monkeys (Cebus apella) Cognition. 2011;120:90–105. doi: 10.1016/j.cognition.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Smith JD, Coutinho MVC, Couchman JJ, Boomer J. The psychological organization of “uncertainty” responses and “middle” responses: A dissociation in capuchin monkeys (Cebus apella) Journal of Experimental Psychology: Animal Behavior Processes. 2009;35:371–381. doi: 10.1037/a0014626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Washburn DA. Chimpanzee responding during matching to sample: Control by exclusion. Journal of the Experimental Analysis of Behavior. 2002;78:497–508. doi: 10.1901/jeab.2002.78-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro D, Matsuzawa T. Numerical ordering in a chimpanzee (Pan troglodytes): Planning, executing, and monitoring. Journal of Comparative Psychology. 1999;113:178–185. [Google Scholar]
- Call J, Carpenter M. Do apes and children know what they have seen? Animal Cognition. 2001;4:207–220. [Google Scholar]
- Chappell J, Kacelnik A. Tool selectivity in a non-primate, the New Caladonian crow (Corvus moneduloides) Animal Cognition. 2002;5:71–78. doi: 10.1007/s10071-002-0130-2. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Dally J, Gilbert J, Dickinson A. Food caching by western scrub jays (Aphelocoma californica) is sensitive to the conditions at recovery. Journal of Experimental Psychology: Animal Behavior Processes. 2005;31:115–124. doi: 10.1037/0097-7403.31.2.115. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Dickinson A. Episodic-like memory during cache recovery by scrub jays. Nature. 1998;395:272–274. doi: 10.1038/26216. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Dickinson A. Scrub jays (Aphelocoma coerulescens) remember the relative time of caching as well as the location and content of their caches. Journal of Comparative Psychology. 1999;113:403–416. doi: 10.1037/0735-7036.113.4.403. [DOI] [PubMed] [Google Scholar]
- Crystal JD. Remembering the past and planning for the future in rats. Behavioural Processes. 2013;93:39–49. doi: 10.1016/j.beproc.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato MR, Colombo M. Representation of serial order in monkeys (Cebus apella) Journal of the Experimental Analysis of Behavior. 1988;14:131–139. [PubMed] [Google Scholar]
- D’Amato MR, Colombo M. Serial learning with wild card items by monkeys (Cebus apella): Implications for knowledge of ordinal position. Journal of Comparative Psychology. 1989;103:252–161. doi: 10.1037/0735-7036.103.3.252. [DOI] [PubMed] [Google Scholar]
- Deaner RO, Isler K, Burkart J, van Schaik C. Overall brain size, and not encephalization quotient, best predicts cognitive ability across non-human primates. Brain, Behavior, and Evolution. 2007;70:115–124. doi: 10.1159/000102973. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Easton A, Zinkivskay A. Recollection in an episodic-like memory task in the rat. Learning and Memory. 2008;12:221–223. doi: 10.1101/lm.92505. [DOI] [PubMed] [Google Scholar]
- Evans TA, Beran MJ. Monkeys exhibit prospective memory in a computerized task. Cognition. 2012;125:131–140. doi: 10.1016/j.cognition.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TA, Beran MJ. Monkeys wait to begin a computer task when waiting makes their responses more effective. Animal Behavior and Cognition. 2014;1:36–50. [Google Scholar]
- Evans TA, Beran MJ, Chan B, Klein ED, Menzel CR. An efficient computerized testing method for the capuchin monkey (Cebus apella): Adaptation of the LRC-CTS to a socially housed nonhuman primate species. Behavior Research Methods. 2008;40:590–596. doi: 10.3758/brm.40.2.590. [DOI] [PubMed] [Google Scholar]
- Evans TA, Perdue BM, Parrish AE, Beran MJ. Working and waiting for better rewards: Self-control in two monkey species (Cebus apella and Macaca mulatta) Behavioural Processes. 2014;103:236–242. doi: 10.1016/j.beproc.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fooden J. Systematic review of the rhesus macaque, Macaca mulatta. Fieldiana Zoology. 2000;96:1–180. [Google Scholar]
- Fragaszy D, Johnson-Pynn J, Hirsh E, Brakke K. Strategic navigation of two-dimensional alley mazes: Comparing capuchin monkeys and chimpanzees. Animal Cognition. 2003;6:149–160. doi: 10.1007/s10071-002-0137-8. [DOI] [PubMed] [Google Scholar]
- Fragaszy D, Kennedy EH, Murnane A, Menzel CR, Brewer G, Johnson-Pynn J, Hopkins WD. Navigating two-dimensional mazes: Chimpanzees (Pan troglodytes) and capuchins (Cebus apella sp.) profit from experience differently. Animal Cognition. 2009;12:491–504. doi: 10.1007/s10071-008-0210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton RR, Zivin A, Murray EA. Rhesus monkeys (Macaca mulatta) discriminate between knowing and not knowing and collect information as needed before acting. Animal Cognition. 2004;7:239–246. doi: 10.1007/s10071-004-0215-1. [DOI] [PubMed] [Google Scholar]
- Inoue S, Matsuzawa T. Working memory of numerals in chimpanzees. Current Biology. 2007;17:R1004–R1005. doi: 10.1016/j.cub.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Iversen IH, Matsuzawa T. Acquisition of navigation by chimpanzees (Pan troglodytes) in an automated fingermaze task. Animal Cognition. 2001;4:179–192. doi: 10.1007/s100710100101. [DOI] [PubMed] [Google Scholar]
- Klein ED, Evans TA, Schultz NB, Beran MJ. Learning how to “make a deal”: Human and monkey performance when repeatedly faced with the Monty Hall Dilemma. Journal of Comparative Psychology. 2013;127:103–108. doi: 10.1037/a0029057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack T, Atance CM. Planning in young children: A review and synthesis. Developmental Review. 2011;31:1–31. [Google Scholar]
- McDaniel MA, Einstein GO. Prospective memory. Los Angeles: Sage Publications; 2007. [Google Scholar]
- Menzel CR. Unprompted recall and reporting of hidden objects by a chimpanzee (Pan troglodytes) after extended delays. Journal of Comparative Psychology. 1999;113:426–434. doi: 10.1037/0735-7036.113.4.426. [DOI] [PubMed] [Google Scholar]
- Menzel EW, Jr, Menzel CR. Do primates plan routes? Simple detour problems reconsidered. In: Washburn D, editor. Primate perspectives on behavior and cognition. Washibngton, DC: American Psychological Association; 2007. pp. 175–206. [Google Scholar]
- Miller GA, Galanter E, Pribram KH. Plans and the structure of behavior. New York: Adams, Bannister, & Cox; 1986. [Google Scholar]
- Miyata H, Fujita K. Pigeons (Columba livia) plan future moves on computerized maze tasks. Animal Cognition. 2008;11:505–516. doi: 10.1007/s10071-008-0141-8. [DOI] [PubMed] [Google Scholar]
- Miyata H, Itakura S, Fujita K. Planning in human children (Homo sapiens) assessed by maze problems on the touchscreen. Journal of Comparative Psychology. 2009;123:69–78. doi: 10.1037/a0012890. [DOI] [PubMed] [Google Scholar]
- Mulcahy NJ, Call J. Apes save tools for future use. Science. 2006 May 19;312:1038–1040. doi: 10.1126/science.1125456. [DOI] [PubMed] [Google Scholar]
- Mushiake H, Saito N, Sakamoto K, Sato Y, Tanji J. Visually based path-planning by Japanese monkeys. Cognitive Brain Research. 2001;11:165–169. doi: 10.1016/s0926-6410(00)00067-7. [DOI] [PubMed] [Google Scholar]
- Naqshbandi M, Roberts WA. Anticipation of future events in squirrel monkeys (Saimiri sciureus) and rats (Ratus norvegicus): Tests of the Bischof-Köhler hypothesis. Journal of Comparative Psychology. 2006;120:345–357. doi: 10.1037/0735-7036.120.4.34. [DOI] [PubMed] [Google Scholar]
- Osvath M, Osvath H. Chimpanzee (Pan troglodytes) and orangutan (Pongo abelii) forethought: Self-control and pre-experience in the face of future tool use. Animal Cognition. 2008;11:661–674. doi: 10.1007/s10071-008-0157-0. [DOI] [PubMed] [Google Scholar]
- Pan J, Kennedy EH, Pickering T, Menzel CR, Stone BW, Fragaszy DM. Development of maze navigation by tufted capuchins (Cebus apella) Behavioral and Brain Sciences. 2011;86:206–215. doi: 10.1016/j.beproc.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raby CR, Alexis DM, Dickinson A, Clayton NS. Planning for the future by western scrub-jays. Nature. 2007;445:919–921. doi: 10.1038/nature05575. [DOI] [PubMed] [Google Scholar]
- Richardson WK, Washburn DA, Hopkins WD, Savage-Rumbaugh ES, Rumbaugh DM. The NASA/LRC Computerized Test System. Behavior Research Methods, Instruments, and Computers. 1990;22:127–131. doi: 10.3758/bf03203132. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Insel TR. The primate neocortex in comparative perspective using magnetic resonance imaging. Journal of Human Evolution. 1999;37:191–223. doi: 10.1006/jhev.1999.0313. [DOI] [PubMed] [Google Scholar]
- Roberts WA. Are animals stuck in time? Psychological Bulletin. 2002;128:473–489. doi: 10.1037/0033-2909.128.3.473. [DOI] [PubMed] [Google Scholar]
- Rumbaugh DM, Pate JL. The evolution of cognition in primates: A comparative perspective. In: Roitblat HL, Bever TG, Terrace HS, editors. Animal Cognition. Hillsdale, NJ: Erlbaum; 1984. pp. 569–587. [Google Scholar]
- Scarf D, Colombo M. Eye movements during list execution reveal no planning in monkeys (Macaca fascicularis) Journal of Experimental Psychology: Animal Behavior Processes. 2009;35:587–592. doi: 10.1037/a0014020. [DOI] [PubMed] [Google Scholar]
- Scarf D, Colombo M. The formation and execution of sequential plans in pigeons (Columba livia) Behavioural Processes. 2010;83:179–182. doi: 10.1016/j.beproc.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Scarf D, Danly E, Morgan G, Colombo M, Terrace HS. Sequential planning in rhesus monkeys (Macaca mulatta) Animal Cognition. 2011;14:317–324. doi: 10.1007/s10071-010-0365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarf D, Terrace H, Colombo M, Magnuson JS. Eye movements reveal planning in humans: A comparison with Scarf and Colombo’s (2009) monkeys. Journal of Experimental Psychology: Animal Learning and Cognition. 2014;40:178–184. doi: 10.1037/xan0000008. [DOI] [PubMed] [Google Scholar]
- Suddendorf T, Busby J. Mental time travel in animals? Trends in Cognitive Sciences. 2003;7:391–396. doi: 10.1016/s1364-6613(03)00187-6. [DOI] [PubMed] [Google Scholar]
- Suddendorf T, Corballis MC. The evolution of foresight: What is mental time travel, and is it unique to humans? Behavioral and Brain Sciences. 2007;30:299–351. doi: 10.1017/S0140525X07001975. [DOI] [PubMed] [Google Scholar]
- Tecwyn EC, Thorpe SKS, Chappell J. A novel test of planning ability: Great apes can plan step-by-step but not in advance of action. Behavioural Processes. 2013;100:174–184. doi: 10.1016/j.beproc.2013.09.016. [DOI] [PubMed] [Google Scholar]
- Tecwyn EC, Thorpe SKS, Chappell J. Development of planning in 4- to 10- year-old children: Reducing inhibitory demands does not improve performance. Journal of Experimental Child Psychology. 2014;125:85–101. doi: 10.1016/j.jecp.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Terrace HS. A nonverbal organism’s knowledge of ordinal position in a serial learning task. Journal of Experimental Psychology: Animal Behavior Processes. 1986;12:203–214. [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson E, editors. Organization of memory. San Diego, CA: Academic Press; 1972. pp. 381–403. [Google Scholar]
- Tulving E. What is episodic memory? Current Directions in Psychological Science. 1993;2:67–70. [Google Scholar]
- Tulving E. Episodic memory and autonoesis: Uniquely human? In: Terrace H, Metcalfe J, editors. The missing link in cognition: Evolution of self-knowing consciousness. New York: Oxford University Press; 2005. pp. 3–56. [Google Scholar]
- Völter CJ, Call J. Younger apes and human children plan their moves in a maze task. Cognition. 2014a;130:186–203. doi: 10.1016/j.cognition.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Völter CJ, Call J. The cognitive underpinnings of flexible tool use in great apes. Journal of Experimental Psychology: Animal Learning and Cognition. 2014b;40:287–302. doi: 10.1037/xan0000025. [DOI] [PubMed] [Google Scholar]
- Washburn DA. Analyzing the path of responding in maze-solving and other tasks. Behavior Reseach Methods, Instruments, & Computers. 1992;24:248–252. doi: 10.3758/bf03203502. [DOI] [PubMed] [Google Scholar]
- Wilson AG, Crystal JD. Prospective memory in the rat. Animal Cognition. 2012;15:349–358. doi: 10.1007/s10071-011-0459-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AG, Pizzo MJ, Crystal JD. Event-based prospective memory in the rat. Current Biology. 2013;23:1089–1093. doi: 10.1016/j.cub.2013.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentall TR. Animals may not be stuck in time. Learning and Motivation. 2005;36:208–225. [Google Scholar]