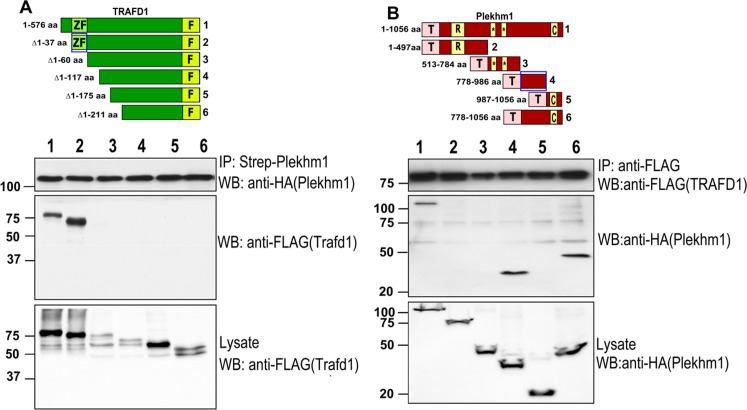
Fig 1. Binding of Plekhm1 with TRAFD1.
(A) Pull-down of full-length, N-terminally TAP-tagged (streptavidin binding protein:HA:calmodulin binding protein) Plekhm1 with TRAFD1-FLAG constructs overexpressed in HEK293 cells. Numbers of constructs in upper panel correspond to lanes in lower panel. Lysates were pulled down with streptavidin-Sepharose followed by western blotting with anti-FLAG (middle blot). The same membrane was probed with anti-HA antibody to monitor pull-down efficiency of Plekhm1 (upper blot). A fraction of the lysate was also probed with anti-FLAG to control for TRAFD1 protein expression (lower blot). Amino acids 37–60 (lane 2 and blue box), containing the zinc-finger, were required for binding to Plekhm1 (lane 2). (B) Reciprocal experiments were done with Plekhm1 constructs diagrammed in the upper panel, with numbers corresponding to lanes in the blots in the lower panel. IP of C-terminally FLAG-tagged full-length TRAFD1 was done with TAP-tagged Plekhm1 constructs. Lysates were immunoprecipitated with anti-FLAG-agarose, blotted, and probed with anti-HA to detect Plekhm1 (middle blot). The same membrane was stripped and probed with monoclonal anti-FLAG antibody to monitor immunoprecipitation efficiency (upper blot). A fraction of the lysate was also probed with anti-HA to control for Plekhm1 protein expression (lower blot). Amino acids 784–986 (of 1059 total), between the second PH domain and the C1 domain, were required for binding TRAFD1 (lane 4 and blue box). The experiments were performed at least 3 times and representative gels are shown. Numbers on left show positions of molecular weight markers. ZF = zinc finger; F = FLAG tag; T = TAP tag; R = RUN domain; * = PH domains; C = C1 domain.