Table 1.
16 Studies of Teams Involved in Cancer Care
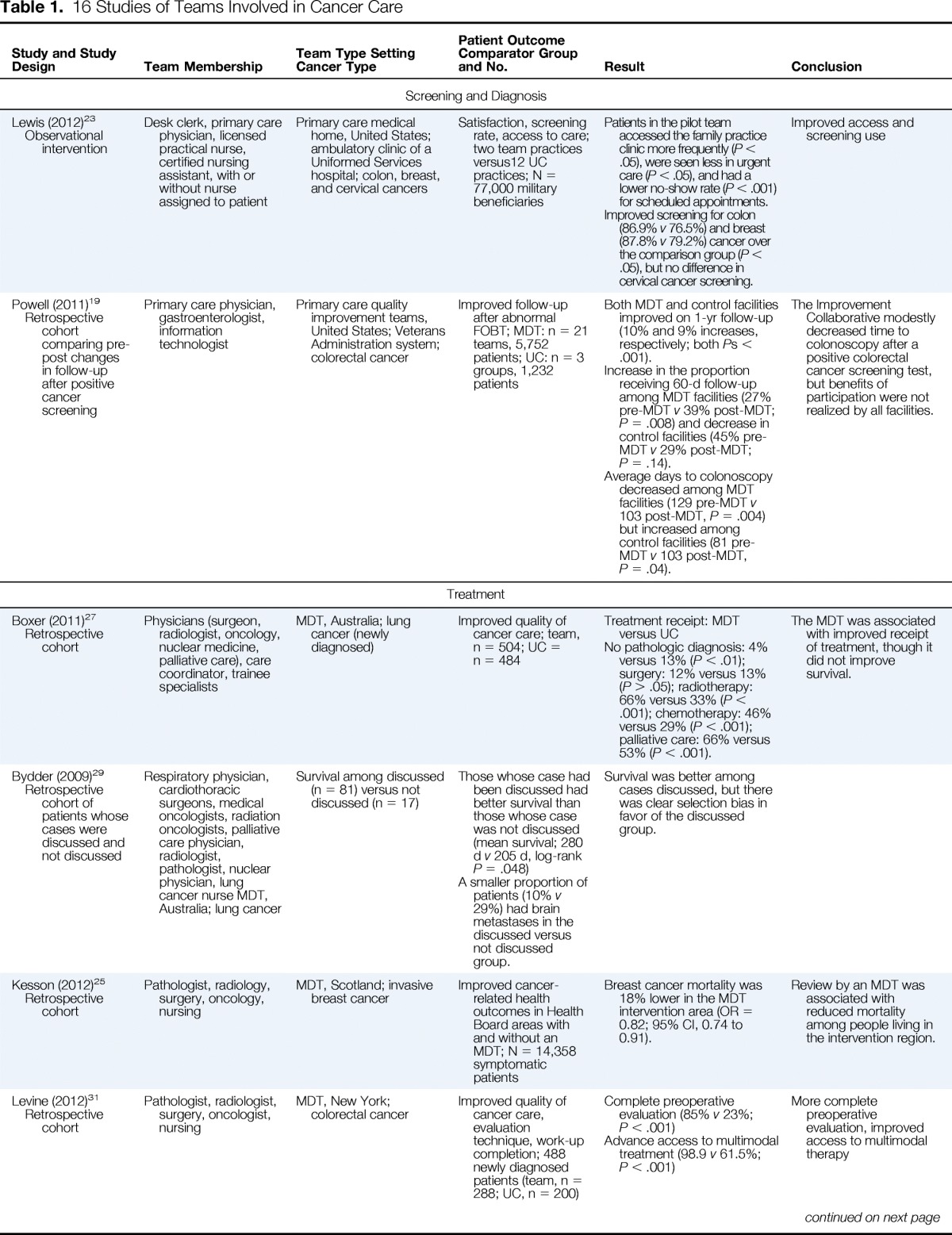
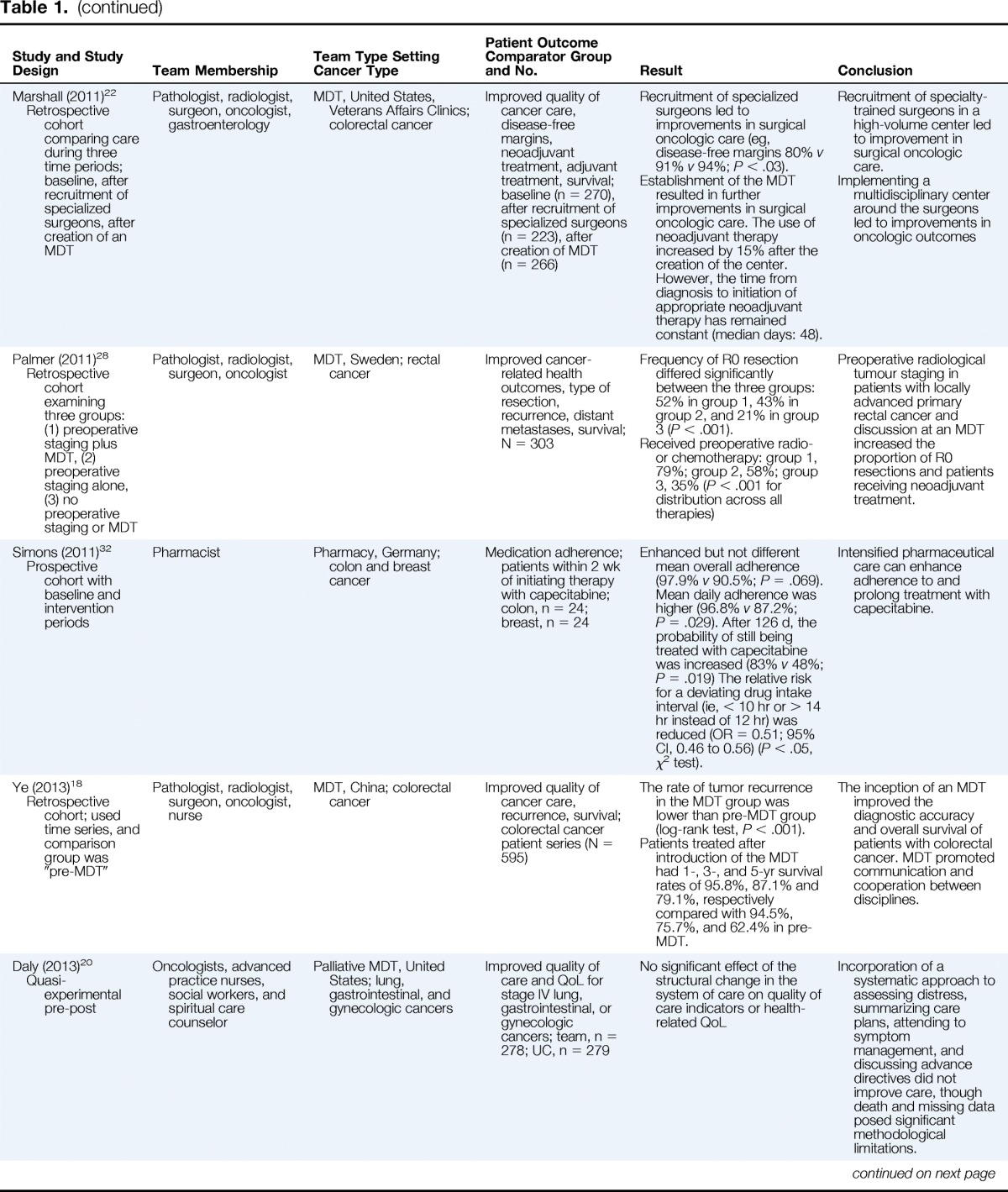
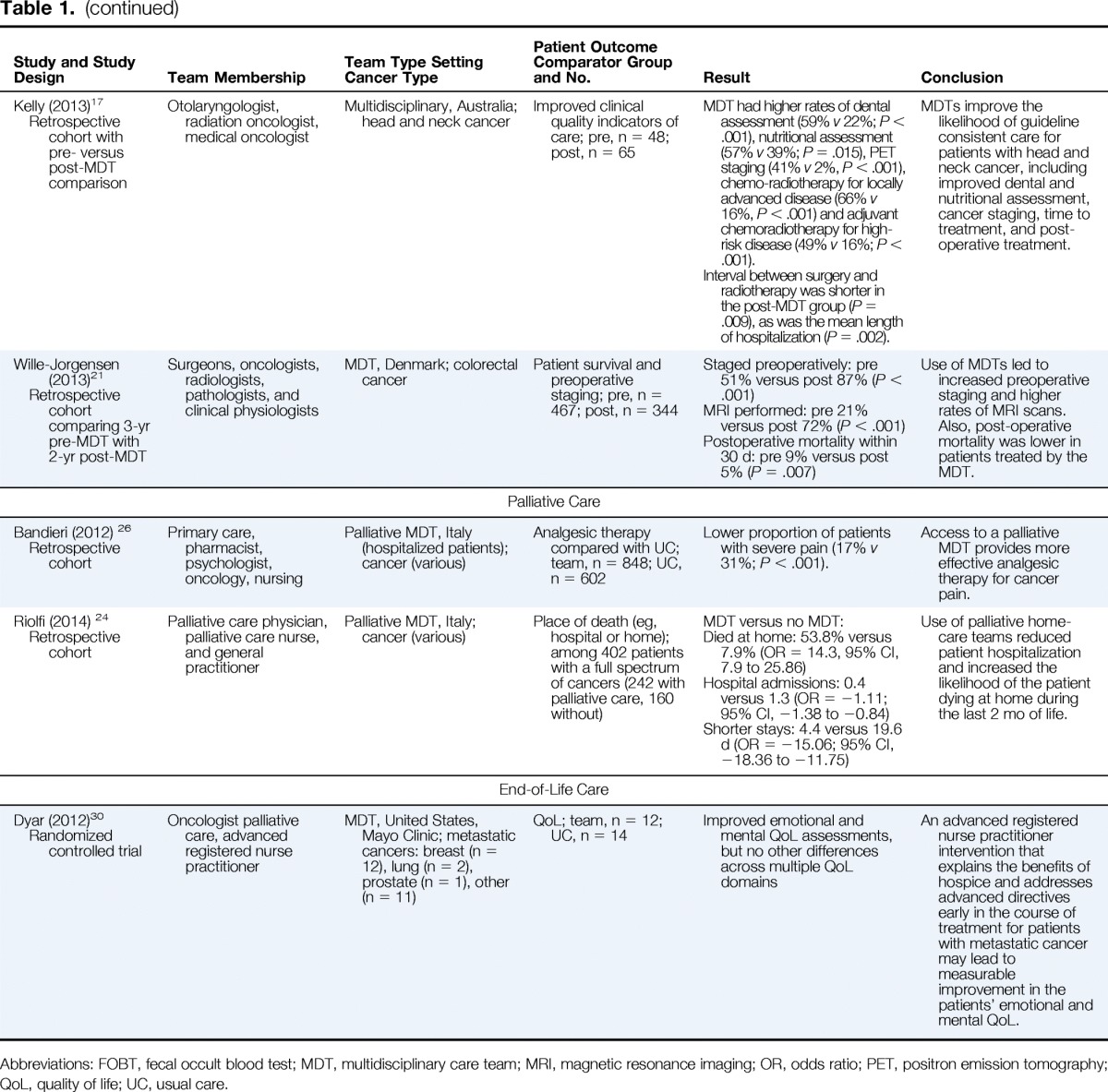
| Study and Study Design | Team Membership | Team Type Setting Cancer Type | Patient Outcome Comparator Group and No. | Result | Conclusion |
|---|---|---|---|---|---|
| Screening and Diagnosis | |||||
| Lewis (2012)23 Observational intervention |
Desk clerk, primary care physician, licensed practical nurse, certified nursing assistant, with or without nurse assigned to patient | Primary care medical home, United States; ambulatory clinic of a Uniformed Services hospital; colon, breast, and cervical cancers | Satisfaction, screening rate, access to care; two team practices versus12 UC practices; N = 77,000 military beneficiaries | Patients in the pilot team accessed the family practice clinic more frequently (P < .05), were seen less in urgent care (P < .05), and had a lower no-show rate (P < .001) for scheduled appointments. Improved screening for colon (86.9% v 76.5%) and breast (87.8% v 79.2%) cancer over the comparison group (P < .05), but no difference in cervical cancer screening. |
Improved access and screening use |
| Powell (2011)19 Retrospective cohort comparing pre-post changes in follow-up after positive cancer screening |
Primary care physician, gastroenterologist, information technologist | Primary care quality improvement teams, United States; Veterans Administration system; colorectal cancer | Improved follow-up after abnormal FOBT; MDT: n = 21 teams, 5,752 patients; UC: n = 3 groups, 1,232 patients | Both MDT and control facilities improved on 1-yr follow-up (10% and 9% increases, respectively; both Ps < .001). Increase in the proportion receiving 60-d follow-up among MDT facilities (27% pre-MDT v 39% post-MDT; P = .008) and decrease in control facilities (45% pre-MDT v 29% post-MDT; P = .14). Average days to colonoscopy decreased among MDT facilities (129 pre-MDT v 103 post-MDT, P = .004) but increased among control facilities (81 pre-MDT v 103 post-MDT, P = .04). |
The Improvement Collaborative modestly decreased time to colonoscopy after a positive colorectal cancer screening test, but benefits of participation were not realized by all facilities. |
| Treatment | |||||
| Boxer (2011)27 Retrospective cohort |
Physicians (surgeon, radiologist, oncology, nuclear medicine, palliative care), care coordinator, trainee specialists | MDT, Australia; lung cancer (newly diagnosed) | Improved quality of cancer care; team, n = 504; UC = n = 484 | Treatment receipt: MDT versus UC No pathologic diagnosis: 4% versus 13% (P < .01); surgery: 12% versus 13% (P > .05); radiotherapy: 66% versus 33% (P < .001); chemotherapy: 46% versus 29% (P < .001); palliative care: 66% versus 53% (P < .001). |
The MDT was associated with improved receipt of treatment, though it did not improve survival. |
| Bydder (2009)29 Retrospective cohort of patients whose cases were discussed and not discussed |
Respiratory physician, cardiothoracic surgeons, medical oncologists, radiation oncologists, palliative care physician, radiologist, pathologist, nuclear physician, lung cancer nurse MDT, Australia; lung cancer | Survival among discussed (n = 81) versus not discussed (n = 17) | Those whose case had been discussed had better survival than those whose case was not discussed (mean survival; 280 d v 205 d, log-rank P = .048) A smaller proportion of patients (10% v 29%) had brain metastases in the discussed versus not discussed group. |
Survival was better among cases discussed, but there was clear selection bias in favor of the discussed group. | |
| Kesson (2012)25 Retrospective cohort |
Pathologist, radiology, surgery, oncology, nursing | MDT, Scotland; invasive breast cancer | Improved cancer-related health outcomes in Health Board areas with and without an MDT; N = 14,358 symptomatic patients | Breast cancer mortality was 18% lower in the MDT intervention area (OR = 0.82; 95% CI, 0.74 to 0.91). | Review by an MDT was associated with reduced mortality among people living in the intervention region. |
| Levine (2012)31 Retrospective cohort |
Pathologist, radiologist, surgery, oncologist, nursing | MDT, New York; colorectal cancer | Improved quality of cancer care, evaluation technique, work-up completion; 488 newly diagnosed patients (team, n = 288; UC, n = 200) | Complete preoperative evaluation (85% v 23%; P < .001) Advance access to multimodal treatment (98.9 v 61.5%; P < .001) |
More complete preoperative evaluation, improved access to multimodal therapy |
| Marshall (2011)22 Retrospective cohort comparing care during three time periods; baseline, after recruitment of specialized surgeons, after creation of an MDT |
Pathologist, radiologist, surgeon, oncologist, gastroenterology | MDT, United States, Veterans Affairs Clinics; colorectal cancer | Improved quality of cancer care, disease-free margins, neoadjuvant treatment, adjuvant treatment, survival; baseline (n = 270), after recruitment of specialized surgeons (n = 223), after creation of MDT (n = 266) | Recruitment of specialized surgeons led to improvements in surgical oncologic care (eg, disease-free margins 80% v 91% v 94%; P < .03). Establishment of the MDT resulted in further improvements in surgical oncologic care. The use of neoadjuvant therapy increased by 15% after the creation of the center. However, the time from diagnosis to initiation of appropriate neoadjuvant therapy has remained constant (median days: 48). |
Recruitment of specialty-trained surgeons in a high-volume center led to improvement in surgical oncologic care. Implementing a multidisciplinary center around the surgeons led to improvements in oncologic outcomes |
| Palmer (2011)28 Retrospective cohort examining three groups: (1) preoperative staging plus MDT, (2) preoperative staging alone, (3) no preoperative staging or MDT |
Pathologist, radiologist, surgeon, oncologist | MDT, Sweden; rectal cancer | Improved cancer-related health outcomes, type of resection, recurrence, distant metastases, survival; N = 303 | Frequency of R0 resection differed significantly between the three groups: 52% in group 1, 43% in group 2, and 21% in group 3 (P < .001). Received preoperative radio- or chemotherapy: group 1, 79%; group 2, 58%; group 3, 35% (P < .001 for distribution across all therapies) |
Preoperative radiological tumour staging in patients with locally advanced primary rectal cancer and discussion at an MDT increased the proportion of R0 resections and patients receiving neoadjuvant treatment. |
| Simons (2011)32 Prospective cohort with baseline and intervention periods |
Pharmacist | Pharmacy, Germany; colon and breast cancer | Medication adherence; patients within 2 wk of initiating therapy with capecitabine; colon, n = 24; breast, n = 24 | Enhanced but not different mean overall adherence (97.9% v 90.5%; P = .069). Mean daily adherence was higher (96.8% v 87.2%; P = .029). After 126 d, the probability of still being treated with capecitabine was increased (83% v 48%; P = .019) The relative risk for a deviating drug intake interval (ie, < 10 hr or > 14 hr instead of 12 hr) was reduced (OR = 0.51; 95% CI, 0.46 to 0.56) (P < .05, χ2 test). | Intensified pharmaceutical care can enhance adherence to and prolong treatment with capecitabine. |
| Ye (2013)18 Retrospective cohort; used time series, and comparison group was “pre-MDT” |
Pathologist, radiologist, surgeon, oncologist, nurse | MDT, China; colorectal cancer | Improved quality of cancer care, recurrence, survival; colorectal cancer patient series (N = 595) | The rate of tumor recurrence in the MDT group was lower than pre-MDT group (log-rank test, P < .001). Patients treated after introduction of the MDT had 1-, 3-, and 5-yr survival rates of 95.8%, 87.1% and 79.1%, respectively compared with 94.5%, 75.7%, and 62.4% in pre-MDT. |
The inception of an MDT improved the diagnostic accuracy and overall survival of patients with colorectal cancer. MDT promoted communication and cooperation between disciplines. |
| Daly (2013)20 Quasi-experimental pre-post |
Oncologists, advanced practice nurses, social workers, and spiritual care counselor | Palliative MDT, United States; lung, gastrointestinal, and gynecologic cancers | Improved quality of care and QoL for stage IV lung, gastrointestinal, or gynecologic cancers; team, n = 278; UC, n = 279 | No significant effect of the structural change in the system of care on quality of care indicators or health-related QoL | Incorporation of a systematic approach to assessing distress, summarizing care plans, attending to symptom management, and discussing advance directives did not improve care, though death and missing data posed significant methodological limitations. |
| Kelly (2013)17 Retrospective cohort with pre- versus post-MDT comparison |
Otolaryngologist, radiation oncologist, medical oncologist | Multidisciplinary, Australia; head and neck cancer | Improved clinical quality indicators of care; pre, n = 48; post, n = 65 | MDT had higher rates of dental assessment (59% v 22%; P < .001), nutritional assessment (57% v 39%; P = .015), PET staging (41% v 2%, P < .001), chemo-radiotherapy for locally advanced disease (66% v 16%, P < .001) and adjuvant chemoradiotherapy for high-risk disease (49% v 16%; P < .001). Interval between surgery and radiotherapy was shorter in the post-MDT group (P = .009), as was the mean length of hospitalization (P = .002). |
MDTs improve the likelihood of guideline consistent care for patients with head and neck cancer, including improved dental and nutritional assessment, cancer staging, time to treatment, and post-operative treatment. |
| Wille-Jorgensen (2013)21 Retrospective cohort comparing 3-yr pre-MDT with 2-yr post-MDT |
Surgeons, oncologists, radiologists, pathologists, and clinical physiologists | MDT, Denmark; colorectal cancer | Patient survival and preoperative staging; pre, n = 467; post, n = 344 | Staged preoperatively: pre 51% versus post 87% (P < .001) MRI performed: pre 21% versus post 72% (P < .001) Postoperative mortality within 30 d: pre 9% versus post 5% (P = .007) |
Use of MDTs led to increased preoperative staging and higher rates of MRI scans. Also, post-operative mortality was lower in patients treated by the MDT. |
| Palliative Care | |||||
| Bandieri (2012) 26 Retrospective cohort |
Primary care, pharmacist, psychologist, oncology, nursing | Palliative MDT, Italy (hospitalized patients); cancer (various) | Analgesic therapy compared with UC; team, n = 848; UC, n = 602 | Lower proportion of patients with severe pain (17% v 31%; P < .001). | Access to a palliative MDT provides more effective analgesic therapy for cancer pain. |
| Riolfi (2014) 24 Retrospective cohort |
Palliative care physician, palliative care nurse, and general practitioner | Palliative MDT, Italy; cancer (various) | Place of death (eg, hospital or home); among 402 patients with a full spectrum of cancers (242 with palliative care, 160 without) | MDT versus no MDT: Died at home: 53.8% versus 7.9% (OR = 14.3, 95% CI, 7.9 to 25.86) Hospital admissions: 0.4 versus 1.3 (OR = −1.11; 95% CI, −1.38 to −0.84) Shorter stays: 4.4 versus 19.6 d (OR = −15.06; 95% CI, −18.36 to −11.75) |
Use of palliative home-care teams reduced patient hospitalization and increased the likelihood of the patient dying at home during the last 2 mo of life. |
| End-of-Life Care | |||||
| Dyar (2012)30 Randomized controlled trial |
Oncologist palliative care, advanced registered nurse practitioner | MDT, United States, Mayo Clinic; metastatic cancers: breast (n = 12), lung (n = 2), prostate (n = 1), other (n = 11) | QoL; team, n = 12; UC, n = 14 | Improved emotional and mental QoL assessments, but no other differences across multiple QoL domains | An advanced registered nurse practitioner intervention that explains the benefits of hospice and addresses advanced directives early in the course of treatment for patients with metastatic cancer may lead to measurable improvement in the patients' emotional and mental QoL. |
Abbreviations: FOBT, fecal occult blood test; MDT, multidisciplinary care team; MRI, magnetic resonance imaging; OR, odds ratio; PET, positron emission tomography; QoL, quality of life; UC, usual care.
