Abstract
The mechanism of a lower incidence of dermatological manifestations in patients treated with enalapril compared to patients treated with other ACE-inhibitors, e.g., captopril, is not known. The finding that prolidase plays an important role in collagen biosynthesis and that some angiotensin-converting enzyme inhibitors affect prolidase activity led us to evaluate its effect on collagen biosynthesis in cultured human skin fibroblasts. Since insulin-like growth factor (IGF-I) and transforming growth factor beta 1 (TGF-β1) are the most potent stimulators of both collagen biosynthesis and prolidase activity, and prolidase is regulated by β1 integrin signaling, the effect of enalapril and enalaprilat on IGF-IR, TGF-β1, and β1 integrin receptor expressions was evaluated. Cells were treated with milimolar concentrations (0.3 and 0.5 mM) of enalapril and enalaprilat for 24 h. The activity of prolidase was determined by colorimetic assay. Collagen biosynthesis was evaluated by radiometric assay. Expression of signaling proteins was evaluated using Western blot. It was found that enalapril- and enalaprilat-dependent increase in prolidase activity and expression was accompanied by parallel increase in collagen biosynthesis. The exposure of the cells to 0.5 mM enalapril and enalaprilat contributed to increase in IGF-IR and α2β1 integrin receptor as well as TGF-β1 and NF-κB p65 expressions. Enalapril- and enalaprilat-dependent increase of collagen biosynthesis in fibroblasts results from increase of prolidase activity and expression, which may undergo through activation of α2β1 integrin and IGF-IR signaling as well as upregulation of TGF-β1 and NF-κB p65, the inhibitor of collagen gene expression.
Keywords: Enalapril, Enalaprilat, Collagen biosynthesis, Fibroblasts, Prolidase
Introduction
Enalapril, (S)-1-[N-(1-etoxycarbonyl)-3-phenylpropyl]-L-proline, a prodrug, is converted by deesterification to angiotensin-converting enzyme inhibitor (ACE-I), enalaprilat, (S)-1-[N-(1-carboxy-3-phenylpropyl)-L-alanyl]-L-proline dihydrate, commonly used to control hypertension (Ovchinnikov et al. 2009). It was the first dicarboxylate-containing ACE inhibitor, which was developed to overcome the limitations of captopril. The sulfhydryl moiety was replaced by a carboxylate moiety, but additional modifications were required in its structure to achieve a similar potency to captopril.
It was documented that treatment of hypertensive patients with enalaprilat gives a lower incidence of dermatological manifestations than results from other ACE-I treatment—captopril (Davies et al. 1984). Structurally, enalaprilat is a dipeptide having L-proline as the C-terminal residue. Dipeptides of the x-pro type are substrates exclusively for prolidase. In contrast to captopril, enalaprilat had no inhibitory effect on porcine kidney prolidase (PKP) (King et al. 1989). This dipeptidase is present in all tissues and plays an important role in the recycling of proline from imidodipeptides (derived from degradation products of collagen) for collagen re-synthesis (Yaron and Naider 1993) and cell growth (Emmerson and Phang 1993). The efficiency of recycling of proline was found to be about 90 % (Jackson et al. 1975). It is evident that an absence of prolidase severely impedes the recycling of collagen proline. Some clinical symptoms related to collagen deficit can be attributed to prolidase deficiency (Freij et al. 1984; Cabrera et al. 2004; Lupi et al. 2008). On the other hand, an increased activity of liver prolidase was found during the fibrotic process (Myara et al. 1987). There was a positive correlation between prolidase activity and fibrosis scores in the lung (Türkbeyler et al. 2012). It suggests that the enzyme activity (despite the collagen gene expression) may be a step-limiting factor in regulation of collagen biosynthesis (Surazynski et al. 2008a). A significant increase in serum prolidase activity was observed in patients with hypertension, which was interpreted as evidence of increased collagen degradation with a higher collagen turnover rate in hypertension tissues, contributing to left ventricular hypertrophy (Demirbag et al. 2007).
Collagen, which accounts for about one third of total body proteins, is not only essential for the maintenance of connective tissue architecture. The interaction between cells and extracellular matrix (ECM) proteins, e.g., collagen, can regulate cellular gene expression, differentiation, and growth (Bissel 1981; Carey 1991). The interaction is mediated by specific cell surface receptors of integrin family. The α2β1 integrin is known as a main collagen receptor. Activation of this receptor by collagen ligation initiates cascade of signaling pathway including FAK, Src, Shc, Grb2, Sos, Ras, Raf and MAP kinases, ERK1, and ERK2 (Boudreau and Jones 1999). Decrease in collagen availability for integrin receptor interaction may therefore potentially alter cellular metabolism. Prolidase activity is stimulated through a signal mediated by collagen–β1 integrin receptor interaction (Palka and Phang 1997, 1998). This pathway is known to be involved in phosphorylation of several intracellular proteins, including prolidase (Surazynski et al. 2001).
Another important point of collagen biosynthesis regulation is at the level of insulin-like growth factor-I receptor (IGF-IR). IGF-I is one of the potent collagen-stimulating factor in collagen-synthesizing cells (Goldstein et al. 1989). Stimulated IGF-I receptor induces interaction of several signaling proteins, such as Grb2, Src, and Shc. This interaction allows activating further cascade of signaling pathway through Sos, Ras, and Raf proteins and, subsequently, two MAP kinases—ERK1 and ERK2 (Werner and Le Roith 2000). The end point of this phenomenon is induction of some transcription factors that regulate cellular metabolism. Some of these activities are regulated through NF-κB, the known inhibitor of collagen gene expression (Kouba et al. 1999). On the other hand, transforming growth factor beta 1 (TGF-β1) may take part in the stimulation of collagen biosynthesis (Surazynski et al. 2010). It was documented in studies where anti-TGF-β1 antibody or gene silencing by si-TGF-β1 counteracted TGF-β1-dependent increase in collagen type I production (Jiang et al. 2012).
Considering the above mentioned factors, we studied the cellular mechanisms for the effect of enalapril-prodrug and enalaprilat-active form of the drug on collagen biosynthesis in cultured human dermal fibroblasts.
Materials and methods
Alkaline phosphatase-labeled anti-mouse IgG, anti-rabbit IgG, and anti-goat IgG antibodies, bacterial collagenase, enalapril maleate salt and enalaprilat dihydrate (dissolved in DMEM), Fast BCIP/NBT reagent, L-glycyl-proline, L-proline, monoclonal (rabbit) TGF-β1 antibody, and monoclonal (mouse) anti-IGF-IR antibody were provided by Sigma Corp., USA., as were most other chemicals and buffers used. Dulbecco’s minimal essential medium (DMEM) and fetal bovine serum (FBS) used in cell culture were products of Gibco, USA. Glutamine, penicillin, and streptomycin were obtained from Quality Biologicals Inc., USA. Nitrocellulose membrane (0.2 μm), sodium dodecylsulphate (SDS), polyacrylamide, molecular weight standards, and Coomassie Briliant Blue R-250 were received from Bio-Rad Laboratories, USA. L-5[3H] proline (28 Ci/mmol) was purchased from Amersham, UK. Monoclonal (mouse) anti-β1, polyclonal (rabbit) anti-α2-integrin and NF-κB p65 antibodies, and polyclonal (goat) anti-β-actin antibody were the products of Santa Cruz Biotechnology Inc., USA. Polyclonal anti-human prolidase antibody was donated by Dr. James Phang (NCI-Frederick Cancer Research and Development Center, Frederick, MD, USA).
Tissue culture
All studies were performed on normal human skin fibroblasts (CRL-1474), which were purchased from American Type Culture Collection, Manassas, VA, USA. The cells were maintained in DMEM supplemented with 10 % fetal bovine serum (FBS), 2 mmol/l glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin at 37 °C in a 5 % CO2 incubator. Cells were counted in hemocytometer and cultured at 1 × 105 cells per well in 2 ml of growth medium in six-well plates (Costar). Cells reached confluence at day 6, and in most cases, such cells were used for assays. Cells were used in the 8th to 14th passages.
Determination of prolidase activity
The activity of prolidase was determined according to the method of Myara et al. (1982). Protein concentration was measured by the method of Lowry et al. (1951). Enzyme activity was reported as nanomoles of proline released from synthetic substrate, during one minute per milligram of supernatant protein of cell homogenate.
Collagen production
Incorporation of radioactive precursor into proteins was measured after labeling of confluent cells in growth medium with enalapril-prodrug and enalaprilat-active form for 24 h with 5[3H] proline (5 μCi/ml, 28 Ci/mM) as described previously (Oyamada et al. 1990). Incorporation of tracer into collagen was determined by digesting proteins with purified Clostridium histolyticum collagenase, according to the method of Peterkofsky et al. (1982). Results are shown as combined values for cell plus medium fractions.
SDS-PAGE
Slab SDS-polyacrylamide gel electrophoresis (PAGE) was used, according to the method of Laemmli (1970), by using 10 % SDS-polyacrylamide gel.
Western immunoblot analysis
After SDS-PAGE, the gels were allowed to equilibrate for 5 min in 25 mmol/l Tris and 0.2 mol/l glycine in 20 % (v/v) methanol. The protein was transferred to 0.2-μm pore-sized nitrocellulose at 100 mA for 1 h by using a LKB 2117 Multiphor II electrophoresis unit. The nitrocellulose was incubated with the following: monoclonal anti-β1, polyclonal anti-α2-integrin, and NF-κB p65 antibodies at concentration 1:1000; polyclonal antibody against β-actin at concentration 1:3000; polyclonal antibody against prolidase at concentration 1:5000; and monoclonal antibodies against IGF-IR and TGF-β1 at concentration 1:1000 in 5 % dried milk in TBS-T (20 mmol/l Tris-HCl buffer, pH 7.4, containing 150 mmol/l NaCl and 0.05 % Tween 20) for 1 h. In order to analyze β1 integrin subunit and IGF-IR second antibody-alkaline phosphatase conjugated, anti-mouse IgG (whole molecule) was added at concentration 1:7500 in TBS-T; in order to analyze prolidase, α2 integrin subunit, TGF-β1, and NF-κB p65 second antibody alkaline phosphatase conjugated, anti-rabbit IgG (whole molecule) was added at concentration 1:5000; and, in order to analyze β-actin second antibody-alkaline phosphatase conjugated, anti-goat IgG (whole molecule) was added at concentration 1:5000 in TBS-T and incubated for 30 min with slow shaking. Then, nitrocellulose was washed with TBS-T (5 × 5 min) and submitted to Sigma-Fast BCIP/NBT reagent. The intensity of the bands was quantified by densitometric analysis.
Statistics
In all experiments, the mean values for three independent experiments done in duplicates ± standard deviation (SD) were calculated. The results were submitted to statistical analysis using one- way ANOVA followed by Tukey test, accepting *P < 0.05 as significant versus control.
Results
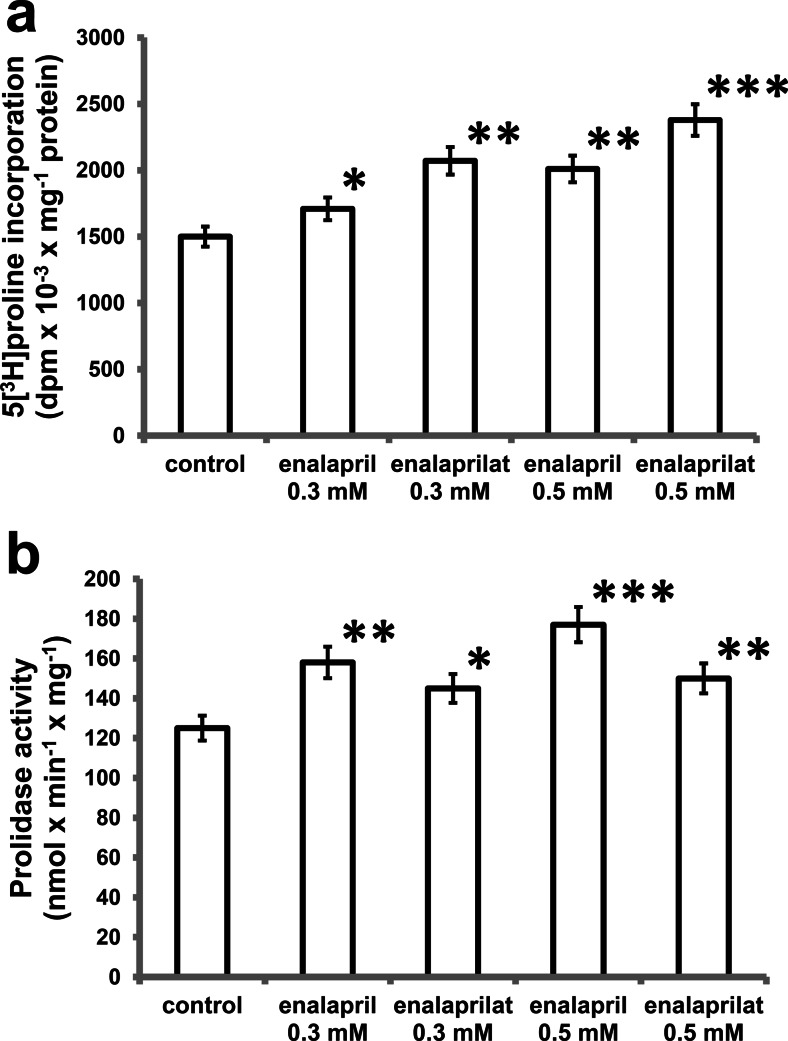
Collagen biosynthesis and prolidase activity were measured in confluent human dermal fibroblasts, which have been treated with 0.3 and 0.5 mM enalapril and enalaprilat (dissolved in DMEM). As can be seen in Fig. 2, 24-h incubation of confluent fibroblasts in the medium containing 10 % of FBS and different concentrations of enalapril or enalaprilat contributed to increase in collagen biosynthesis (Fig. 1a) and prolidase activity (Fig. 1b) in a dose-dependent manner. At 0.3 and 0.5 mM, enalapril induced increase in collagen biosynthesis to about 114 and 134 %, respectively. After 24-h incubation, enalaprilat at 0.3 and 0.5 mM contributed also to increase in collagen biosynthesis to about 138 and 159 %, respectively (Fig. 1a).
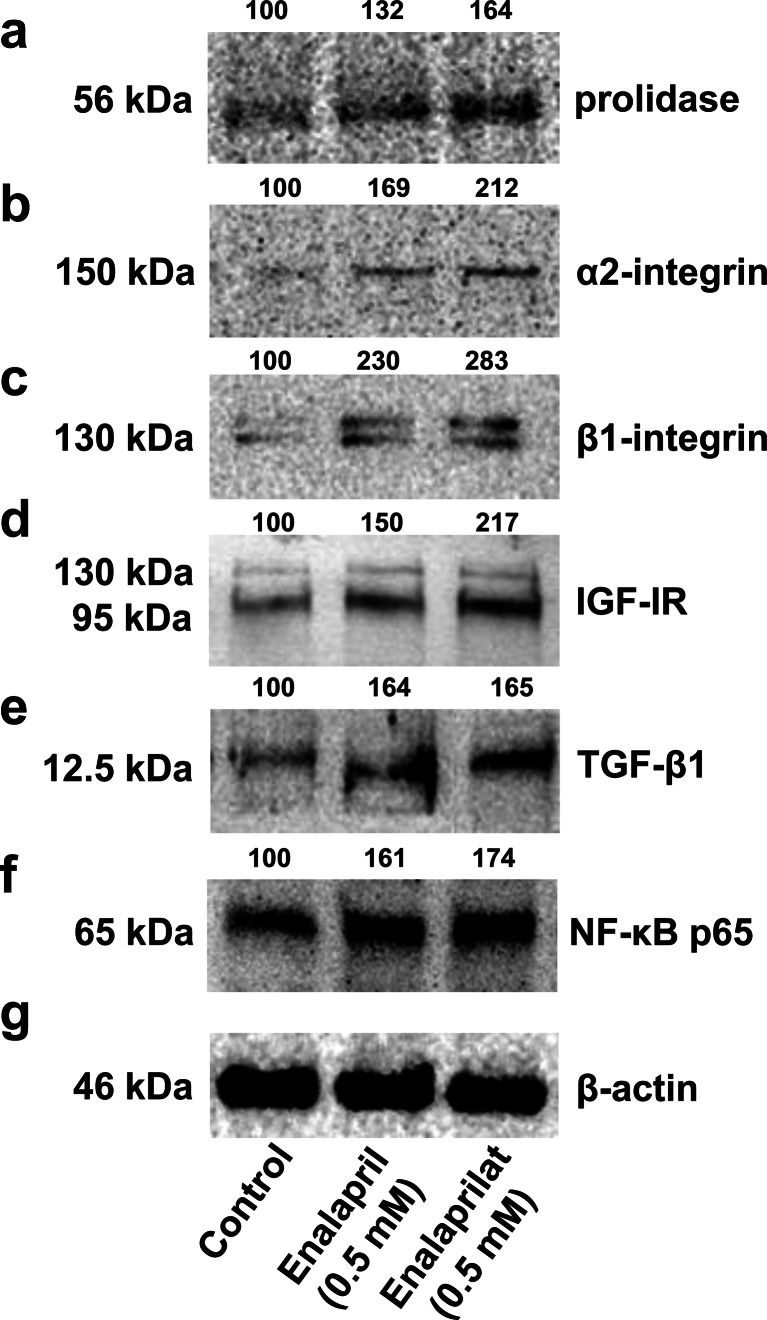
Fig. 2.
Western blot analysis for prolidase (a), α2 integrin receptor (b), β1 integrin receptor (c), IGF receptor (d), TGF-β1 (e), and NF-κB p65 (f) in control human skin fibroblasts (lane 1) and cultured in the medium containing 0.5 mM of enalapril (lane 2) or 0.5 mM of enalaprilat (lane 3). The mean values of six pooled cell homogenate extracts from six separate experiments are presented. The intensity of the bands was quantified by densitometric analysis. Densitometry was done with BioSpectrum Imaging System and presented as an arbitrary units. The same amount of supernatant protein (20 μg) was run in each lane. The expression of β-actin served as a control for protein loading (g)
Fig. 1.
Collagen biosynthesis (a) measured as 5[3H] proline incorporation into proteins susceptible to the action of bacterial collagenase and prolidase activity (b) in confluent human skin fibroblasts incubated for 24 h in the medium containing 10 % FBS and different concentrations of enalapril and enalaprilat. The results present the mean values from six assays ± SD. *P < 0.05; **P < 0.01; ***P < 0.001 compared with the control
Prolidase activity was increased to about 126 and 132 % of control at 0.3 and 0.5 mM of enalapril, respectively. At 0.3 and 0.5 mM, enalaprilat induced prolidase activity to about 128 and 142 % of control, respectively (Fig. 1b).
Increase in prolidase activity due to 24-h treatment of fibroblasts with enalapril or enalaprilat was accompanied by increase in the expression of the enzyme as shown by Western immunoblot analysis (Fig. 2a).
The data shows that both, enalapril and enalaprilat, induced increase in collagen biosynthesis in skin fibroblasts and suggests that the increase may result from activation of prolidase activity and expression.
Collagen biosynthesis and prolidase activity were previously shown to be regulated due to the signal induced by activated α2β1 integrin receptor (Palka and Phang 1997; Ivaska et al. 1999) as well as insulin-like growth factor-I receptor (IGF-IR) (Goldstein et al. 1989) and transforming growth factor beta (TGF beta) (McAnulty et al. 1991; Surazynski et al. 2010). Therefore, the expression of α2β1 integrin receptor (receptor for type I collagen), IGF-IR, and TGF-β1 were measured by Western immunoblot analysis. As can be seen in Fig. 2b, c, 24-h treatment of fibroblasts with 0.5 mM enalapril or enalaprilat contributed to a distinct increase in the expression of α2 and β1 integrin subunits, compared to the control cells (Fig. 2b, c, line 1). In addition, as shown in Fig. 2d, an increase in IGF-I receptor expression was found in enalapril- and enalaprilat-treated cells, compared to control cells. Simultaneously, we have found an increase in the expression of TGF-β1 (Fig. 2e) and NF-κB p65 (Fig. 2f), the known inhibitor of collagen gene expression (Kouba et al. 1999), compared to control cells (Fig. 2e, f, line 1).
In view of this data, it seems that ability of enalapril and enalaprilat to induce collagen biosynthesis may involve increase in prolidase activity and expression, and increase in α2β1 integrin, IGF-I receptor, TGF-β1, and NF-κB p65 expressions.
Discussion
The finding that enalaprilat gives a lower incidence of dermatological manifestations than results from other ACE-I treatment, captopril (Davies et al. 1984), led us to investigate its role in collagen biosynthesis in fibroblasts—the main collagen synthesizing cells (Makela et al. 1990). It is well established that prolidase, providing proline for collagen biosynthesis, is a rate-limiting factor in regulation of this process (Karna et al. 2000, 2001; Galicka et al. 2001; Surazynski et al. 2008a). Therefore, the mechanism of prolidase activity regulation is of considerable interest. In contrast to captopril, enalaprilat had no inhibitory effect on porcine kidney prolidase (PKP) (King et al. 1989). In fact, in our previous studies (Karna et al. 2010), we found that exposure of fibroblasts to captopril contributed to a decrease in prolidase activity, and expression of α2β1 integrin receptor and IGF-IR, suggesting underlying mechanism of captopril-dependent inhibition of collagen biosynthesis. It seems that in view of dermatological manifestation that accompanies captopril therapy, the above mechanism may be of great importance.
In the present study, we found that enalapril and enalaprilat contributed to increase in prolidase activity. Their effect on the activity of other enzymes was also observed (Männistö et al. 2001). The constellation of changes induced by studied ACE inhibitors found in this study suggests an important role of prolidase in upregulation of collagen synthesis. It supports our previous studies on prolidase-dependent regulation of collagen biosynthesis (Karna et al. 2006). Several other studies suggest that prolidase-dependent regulation of collagen biosynthesis may take place at the transcriptional level. The transfection of colorectal cancer cells with prolidase vector inhibited NF-κB expression (Surazynski et al. 2008a), well-recognized inhibitor of expression of α1 and α2 subunits of type I collagen (Kouba et al. 1999; Rippe et al. 1999; Miltyk et al. 2007). Another evidence for the role of prolidase in regulation of NF-κB expression provides experiment showing that inhibition of prolidase activity by Cbz-Pro contributed to upregulation of NF-κB expression in fibroblasts (Surazynski et al. 2008a). In contrast, our data showed that enalapril- and enalaprilat-dependent increase in collagen biosynthesis is accompanied by an increase in the expression of NF-κB p65. Although it is accepted that enalapril inhibits NF-kB-induced inflammatory responses in several experimental “in vivo” models (Kushwaha and Jena 2012), our data on enalapril effect on NF-kB p65 expression in cultured fibroblasts showed contrary results. It cannot be ruled out that in the experimental conditions, enalapril upregulates coordinately expression of both NF-kB and IGF-IR (shown in our studies) since IGF-IR promoter contains NF-kB binding site (Ma et al. 2006). Such a hypothesis seems to be reasonable since recently it was found that enalapril and nifedipine differentially regulate 33 genes involved in the pathogenesis of cardiovascular diseases (Lee et al. 2013).
Enalaprilat as an angiotensin-converting enzyme inhibitor (ACE-I) has a pseudo-peptide structure with a proline residue at the position P′2. This proline residue is also present in the natural substrate of ACE, angiotensin I, and it has been suggested that the preference of proline at position P′2 of the peptide may reside in its rigid conformation that allows the carboxy terminus to be placed in a favorable alignment for the interaction with a positively charged amino acid of the active site of the enzyme. Important role of proline moiety at the binding process was proposed (Andújar-Sánchez et al. 2004).
There are some evidence that ACE-Is increase synthesis of collagen, one of the major constituents of the atherosclerotic cap (Claridge et al. 2004). Rhaleb et al. (2001) found that ACE-Is through indirect mechanism may contribute to regulation of collagen synthesis. We used a range of ACE inhibitors at concentrations up to 0.5 mM because of our previous experience with captopril (Karna et al. 2010). Some studies “in vitro” were performed with higher ACE-I concentration (10 mmol/l) (Mailloux et al. 2003).
The use of ACE-Is in other animal models of atherosclerosis has shown an increase in the extracellular matrix proteins (Rabbani and Topol 1999). These phenomena were explained by the upregulation of collagen synthesis, and the presence of increased quantities of collagen in the fibrous cap (Claridge et al. 2004). Moreover, angiotensinogen, angiotensin-converting enzyme (ACE), and angiotensin II receptor are expressed in skin (Steckelings et al. 2004). It is known that ACE inhibitors might suppress skin disfunction and inflammatory response mediated through the angiotensin II pathway. Treatment with the ACE inhibitor, enalapril, counteracted UVB-induced wrinkles and skin damage (Matsuura-Hachiya et al. 2013).
Some studies have shown diverse effects of different representatives of ACE-Is on tissue collagen metabolism. Perindopril and ramipril prevented overexpression of type IV collagen mRNA in diabetic vessels (Cooper et al. 1998; Gilbert et al. 1998), while quinapril had no effect on collagen type I expression (Hernández-Presa et al. 1998).
Some studies demonstrated that captopril and enalapril improved pulmonary fibrosis in the lung tissue (Ghazi-Khansari et al. 2007). Early application of enalapril following dermal injury reduces formation of hypertrophic scars, probably because of its downregulatory effects on type III collagen production (Uzun et al. 2013). The mechanism of this process may involve TGF-β1 participation (Tang et al. 2009; Jiang et al. 2012; Uzun et al. 2013).
In fact, our data show that enalapril and enalaprilat both increase expression of TGF-β1. Moreover, other authors found that products of catalytic activity of prolidase, Pro and HyPro, induced increase in the amount of TGF-β1 and receptor expression for TGF-β1 (Surazynski et al. 2010).
Collagen is known as a ligand for α2β1 integrin. Previously, it has been shown that α2β1 integrin receptor is involved in signaling, which regulates collagen biosynthesis (Ivaska et al. 1999) and prolidase activity (Palka and Phang 1994, 1997). Another important point of collagen biosynthesis regulation is at the level of insulin-like growth factor-I receptor (IGF-IR). IGF-I is one of the most potent collagen-stimulating factor in collagen-synthesizing cells (Goldstein et al. 1989). Therefore, we considered α2β1 integrin and IGF-IR as a potential target in enalapril- and enalaprilat-induced increase of the above processes.
Our observations suggest that studied drugs increase collagen biosynthesis in fibroblasts primarily through increase of α2β1 integrin receptor and IGF-I-IR expressions. Presumably, increase in prolidase activity in fibroblasts due to enalapril and enalaprilat action is a result of increase of signaling by α2β1 integrin and IGF-IR. Both β1 integrin (Palka and Phang 1997) and IGF-IR (Miltyk et al. 1998) signaling were found to play important role in prolidase activity regulation.
Although several studies on animal models have shown that enalapril and enalaprilat treatment reduces fibrosis in some tissues, it cannot correspond to reduced collagen synthesis. Upregulation of collagen synthesis may reflect interstitial remodeling leading to increase or decrease of tissue collagen content depending on the rate of collagen degradation. It was supported by some studies showing that ACE inhibitors increase type III collagen synthesis (Claridge et al. 2004).
Conclusion
The results of present study suggest that in fibroblasts, enalapril and enalaprilat may exert its effect on collagen biosynthesis through stimulation of prolidase activity and upregulation of expressions of prolidase, α2β1 integrin, IGF-IR, TGF-beta 1, and NF-κB p65.
Acknowledgments
This work was supported by the Medical University of Białystok (Grant No 123-14881F) and conducted with the use of equipment purchased by Medical University of Białystok as part of the OP DEP 2007–2013, Priority Axis I.3, contract No. POPW.01.03.00-20-008/09.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Andújar-Sánchez M, Cámara-Artigas A, Jara-Pérez V. A calorimetric study of the binding of lisinopril, enalaprilat and captopril to angiotensin-converting enzyme. Biophys Chem. 2004;111:183–189. doi: 10.1016/j.bpc.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Bissel M. How does extracellular matrix direct gene expression? J Theor Biol. 1981;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Boudreau NJ, Jones PL. Extracellular matrix and integrin signalling: the shape of things to come. Biochem J. 1999;339:481–488. doi: 10.1042/0264-6021:3390481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera HN, Giovanna PD, Bozzini NF, Forlino A. Prolidase deficiency: case reports of two Argentinian brothers. Int J Dermatol. 2004;43:684–686. doi: 10.1111/j.1365-4632.2004.02411.x. [DOI] [PubMed] [Google Scholar]
- Carey DJ. Control of growth and differentiation of vascular cells by extracellular matrix. Annu Rev Physiol. 1991;53:161–177. doi: 10.1146/annurev.ph.53.030191.001113. [DOI] [PubMed] [Google Scholar]
- Claridge MW, Hobbs SD, Quick CR, Day NE, Bradbury AW, Wilmink AB. ACE inhibitors increase type III collagen synthesis: a potential explanation for reduction in acute vascular events by ACE inhibitors. Eur J Vasc Endovasc Surg. 2004;1:67–70. doi: 10.1016/j.ejvs.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Cooper ME, Cao Z, Rumble JR, Jandeleit K, Allen TJ, Gilbert RE. Attenuation of diabetes-associated mesenteric vascular hypertrophy with perindopril: morphological and molecular biological studies. Metabolism. 1998;12:24–27. doi: 10.1016/S0026-0495(98)90367-5. [DOI] [PubMed] [Google Scholar]
- Davies RO, Irvin JD, Kramsch DK, Walker JF, Moncloa F. Enalapril worldwide experience. Am J Med. 1984;77:23–35. doi: 10.1016/S0002-9343(84)80055-8. [DOI] [PubMed] [Google Scholar]
- Demirbag R, Yildiz A, Gur M, Yilmaz R, Elçi K, Aksoy N. Serum prolidase activity in patients with hypertension and its relation with left ventricular hypertrophy. Clin Biochem. 2007;40:1020–1025. doi: 10.1016/j.clinbiochem.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Emmerson KS, Phang JM. Hydrolysis of proline dipeptides completely fulfills the proline requirement in a proline - auxotropic Chinese Hamster Ovary cell line. J Nutr. 1993;123:909–914. doi: 10.1093/jn/123.5.909. [DOI] [PubMed] [Google Scholar]
- Freij BJ, Levy HL, Dudin G, Mutasim D, Deeb M, Der Kaloustian VM. Clinical and biochemical characteristics of prolidase deficiency in siblings. Am J Med Genet. 1984;19:561–571. doi: 10.1002/ajmg.1320190319. [DOI] [PubMed] [Google Scholar]
- Galicka A, Wołczynski S, Anchim T, Surazynski A, Lesniewicz R, Palka J. Defects of type I procollagen metabolism correlated with decrease of prolidase activity in a case of lethal osteogenesis imperfecta. Eur J Biochem. 2001;268:2172–2178. doi: 10.1046/j.1432-1327.2001.02099.x. [DOI] [PubMed] [Google Scholar]
- Ghazi-Khansari M, Mohammadi-Karakani A, Sotoudeh M, Mokhtary P, Pour-Esmaeil E, Maghsoud S. Antifibrotic effect of captopril and enalapril on paraquat-induced lung fibrosis in rats. J Appl Toxicol. 2007;27:342–349. doi: 10.1002/jat.1212. [DOI] [PubMed] [Google Scholar]
- Gilbert RE, Cox A, Wu LL, Allen TJ, Hulthen UL, Jerums G, Cooper ME. Expression of transforming growth factor-beta1 and type IV collagen in the renal tubulointerstitium in experimental diabetes: effects of ACE inhibition. Diabetes. 1998;47:414–422. doi: 10.2337/diabetes.47.3.414. [DOI] [PubMed] [Google Scholar]
- Goldstein RH, Poliks CF, Pilch PF, Smith BD, Fine A. Stimulation of collagen formation by insulin and insulin-like growth factor-I in cultures of human lung fibroblasts. Endocrinology. 1989;124:964–970. doi: 10.1210/endo-124-2-964. [DOI] [PubMed] [Google Scholar]
- Hernández-Presa MA, Bustos C, Ortego M, Tuñón J, Ortega L, Egido J. ACE inhibitor quinapril reduces the arterial expression of NF-kappaB-dependent proinflammatory factors but not of collagen I in a rabbit model of atherosclerosis. Am J Pathol. 1998;153:1825–1837. doi: 10.1016/S0002-9440(10)65697-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivaska J, Käpylä J, Pentikäinen O, Hoffrén AM, Hermonen J, Huttunen P, Johnson MS, Heino J. A peptide inhibiting the collagen binding function of integrin alpha2I domain. J Biol Chem. 1999;274:3513–3521. doi: 10.1074/jbc.274.6.3513. [DOI] [PubMed] [Google Scholar]
- Jackson SH, Dennis AW, Greenberg M. Iminopeptiduria: a genetic defect in recycling of collagen; a method for determining prolidase in erytrocytes. CMA J. 1975;113:759–763. [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Shao L, Wang Q, Dong Y. Repetitive mechanical stretching modulates transforming growth factor-β induced collagen synthesis and apoptosis in human patellar tendon fibroblasts. Biochem Cell Biol. 2012;90:667–674. doi: 10.1139/o2012-024. [DOI] [PubMed] [Google Scholar]
- Karna E, Surazynski A, Palka J. Collagen metabolism disturbances are accompanied by an increase in prolidase activity in lung carcinoma planoepitheliale. Int J Exp Pathol. 2000;81:341–347. doi: 10.1046/j.1365-2613.2000.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karna E, Miltyk W, Wołczynski S, Palka J. The potential mechanism for glutamine-induced collagen biosynthesis in cultured human skin fibroblasts. Comp Biochem Physiol B. 2001;130:23–32. doi: 10.1016/S1096-4959(01)00400-6. [DOI] [PubMed] [Google Scholar]
- Karna E, Miltyk W, Palka JA. Butyrate-induced collagen biosynthesis in cultured fibroblasts is independent on alpha2beta1 integrin signalling and undergoes through IGF-I receptor cascade. Mol Cell Biochem. 2006;286:147–152. doi: 10.1007/s11010-005-9106-2. [DOI] [PubMed] [Google Scholar]
- Karna E, Szoka L, Palka JA. Captopril-dependent inhibition of collagen biosynthesis in cultured fibroblasts. Pharmazie. 2010;65:1–4. [PubMed] [Google Scholar]
- King GF, Crossley MJ, Kuchel PW. Inhibition and active-site modelling of prolidase. Eur J Biochem. 1989;180:377–384. doi: 10.1111/j.1432-1033.1989.tb14659.x. [DOI] [PubMed] [Google Scholar]
- Kouba DJ, Chung KY, Nishiyama T, Vindevoghel L, Kon A, Klement JF, Uitto J, Mauviel A. Nuclear factor-kappa B mediates TNF-alpha inhibitory effect on alpha 2(I) collagen (COL1A2) gene transcription in human dermal fibroblasts. J Immunol. 1999;162:4226–4234. [PubMed] [Google Scholar]
- Kushwaha S, Jena GB. Enalapril reduces germ cell toxicity in streptozotocin-induced diabetic rat: investigation on possible mechanisms. Naunyn Schmiedebergs Arch Pharmacol. 2012;385:111–124. doi: 10.1007/s00210-011-0707-x. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee KM, Kang HA, Ko CB, Oh EH, Park M, Lee HY, Choi HR, Yun CH, Jung WW, Oh JW, Kang HS. Differential gene expression profiles in spontaneously hypertensive rats induced by administration of enalapril and nifedipine. Int J Mol Med. 2013;31:179–187. doi: 10.3892/ijmm.2012.1183. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NI, Farr AL, Randall IR. Protein measurement with the Folin reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lupi A, Tenni R, Rossi A, Cetta G, Forlino A. Human prolidase and prolidase deficiency: an overview on the characterization of the enzyme involved in proline recycling and on the effects of its mutations. Amino Acids. 2008;35:739–752. doi: 10.1007/s00726-008-0055-4. [DOI] [PubMed] [Google Scholar]
- Ma Y, Zhang L, Peng T, Cheng J, Taneja S, Zhang J, Delafontaine P, Du J. Angiotensin II stimulates transcription of insulin-like growth factor I receptor in vascular smooth muscle cells: role of nuclear factor-kappaB. Endocrinology. 2006;147:1256–1263. doi: 10.1210/en.2005-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailloux A, Deslandes B, Vaubourdolle M, Baudin B. Captopril and enalaprilat decrease antioxidant defences in human endothelial cells and are unable to protect against apoptosis. Cell Biol Int. 2003;27:825–830. doi: 10.1016/S1065-6995(03)00162-8. [DOI] [PubMed] [Google Scholar]
- Makela JK, Vuorio T, Vuorio E. Growth-dependent modulation of type I collagen production and mRNA levels in cultured human skin fibroblasts. Biochim Biophys Acta. 1990;1049:171–176. doi: 10.1016/0167-4781(90)90037-3. [DOI] [PubMed] [Google Scholar]
- Matsuura-Hachiya Y, Arai KY, Ozeki R, Kikuta A, Nishiyama T. Angiotensin- converting enzyme inhibitor (enalapril maleate) accelerates recovery of mouse skin from UVB-induced wrinkles. Biochem Biophys Res Commun. 2013;442:38–43. doi: 10.1016/j.bbrc.2013.10.162. [DOI] [PubMed] [Google Scholar]
- Männistö TK, Karvonen KE, Kerola TV, Ryhänen LJ. Inhibitory effect of the angiotensin converting enzyme inhibitors captopril and enalapril on the conversion of procollagen to collagen. J Hypertens. 2001;10:1835–1839. doi: 10.1097/00004872-200110000-00018. [DOI] [PubMed] [Google Scholar]
- McAnulty RJ, Campa JS, Cambrey AD, Laurent GJ. The effect of transforming growth factor beta on rates of procollagen synthesis and degradation in vitro. Biochim Biophys Acta. 1991;1091:231–235. doi: 10.1016/0167-4889(91)90066-7. [DOI] [PubMed] [Google Scholar]
- Miltyk W, Karna E, Wołczyński S, Pałka J. Insulin-like growth factor I- dependent regulation of prolidase activity in cultured human skin fibroblasts. Mol Cell Biochem. 1998;189:177–184. doi: 10.1023/A:1006958116586. [DOI] [PubMed] [Google Scholar]
- Miltyk W, Karna E, Palka JA. Prolidase-independent mechanism of camptothecin-induced inhibition of collagen biosynthesis in cultured human skin fibroblasts. J Biochem. 2007;141:287–292. doi: 10.1093/jb/mvm022. [DOI] [PubMed] [Google Scholar]
- Myara I, Charpentier C, Lemonnier A. Optimal conditions for prolidase assay by proline colorimetric determination: application to imidopeptiduria. Clin Chim Acta. 1982;125:193–205. doi: 10.1016/0009-8981(82)90196-6. [DOI] [PubMed] [Google Scholar]
- Myara I, Miech G, Fabre M, Mangeot M, Lemonnier A. Changes in prolinase and prolidase activity during CCl4 administration inducing liver cytolysis and fibrosis in rat. Brit J Exp Pathol. 1987;68:7–13. [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov AG, Serbul VM, Ageev FT. Effects of renin-angiotensin system blockers on left ventricular hypertrophy and biochemical markers of collagen balance in patients with hypertensive hypertrophy. Ter Arkh. 2009;81:64–71. [PubMed] [Google Scholar]
- Oyamada I, Palka J, Schalk EM, Takeda K, Peterkofsky B. Scorbutic and fasted guinea pig sera contain an insulin-like growth factor I reversible inhibitor of proteoglycan and collagen synthesis in chick embryo chondrocytes and adult human skin fibroblasts. Arch Biochem Biophys. 1990;276:85–93. doi: 10.1016/0003-9861(90)90013-O. [DOI] [PubMed] [Google Scholar]
- Palka JA, Phang JM. Prolidase (PLD) activity in regulated by cell surface-extracellular matrix (ECM) interaction in normal fibroblast and MCF-7 cells. Proc Am Assoc Cancer Res. 1994;35:531. [Google Scholar]
- Palka JA, Phang JM. Prolidase activity in fibroblasts is regulated by interaction of extracellular matrix with cell surface integrin receptors. J Cell Biochem. 1997;67:166–175. doi: 10.1002/(SICI)1097-4644(19971101)67:2<166::AID-JCB2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Palka JA, Phang JM. Prolidase in human breast cancer MCF-7 cells. Cancer Lett. 1998;127:63–70. doi: 10.1016/S0304-3835(98)00011-1. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B, Chojkier M, Bateman J. Determination of collagen synthesis in tissue and cell culture system. In: Fufthmar M, editor. Immunochemistry of the extracellular matrix. Boca Raton: CRC Press; 1982. pp. 19–47. [Google Scholar]
- Rabbani R, Topol EJ. Strategies to achieve coronary arterial plaque stabilization. Cardiovasc Res. 1999;41:402–417. doi: 10.1016/S0008-6363(98)00279-X. [DOI] [PubMed] [Google Scholar]
- Rhaleb NE, Peng H, Harding P, Tayeh M, Lapointe MC, Carreto OA. Effect of N-Acetyl-Seryl-Aspartyl- Proline on DNA and collagen synthesis in rat cardiac fibroblasts. Hypertension. 2001;37:827–832. doi: 10.1161/01.HYP.37.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippe RA, Schrum LW, Stefanovic B, Solís-Herruzo JA, Brenner DA. NF-kappaB inhibits expression of the alpha1(I) collagen gene. DNA Cell Biol. 1999;18:751–761. doi: 10.1089/104454999314890. [DOI] [PubMed] [Google Scholar]
- Steckelings UM, Wollschlager T, Peters J, Henz BM, Hermes B, Artuc M. Human skin: source of and target organ for angiotensin II. Exp Dermatol. 2004;13:148–154. doi: 10.1111/j.0906-6705.2004.0139.x. [DOI] [PubMed] [Google Scholar]
- Surazynski A, Palka J, Wolczynski S. Phosphorylation of prolidase increases the enzyme activity. Mol Cell Biochem. 2001;220:95–101. doi: 10.1023/A:1010849100540. [DOI] [PubMed] [Google Scholar]
- Surazynski A, Miltyk W, Palka J, Phang JM. Prolidase-dependent regulation of collagen biosynthesis. Amino Acids. 2008;35:731–738. doi: 10.1007/s00726-008-0051-8. [DOI] [PubMed] [Google Scholar]
- Surazynski A, Miltyk W, Prokop I, Palka J. Prolidase-dependent regulation of TGF β (corrected) and TGF β receptor expressions in human skin fibroblasts. Eur J Pharmacol. 2010;649:115–119. doi: 10.1016/j.ejphar.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Tang HT, Cheng DS, Jia YT, Ben DF, Ma B, Lv KY, Wei D, Sheng ZY, Xia ZF. Angiotensin II induces type I collagen gene expression in human dermal fibroblasts through an AP-1/TGF-beta1-dependent pathway. Biochem Biophys Res Commun. 2009;385:418–423. doi: 10.1016/j.bbrc.2009.05.081. [DOI] [PubMed] [Google Scholar]
- Türkbeyler I, Demir T, Pehlivan Y, Kaplan DS, Ceribasi AO, Orkmez M, Aksoy N, Taysi S, Kisacik B, Onat AM. Prolidase could act as a diagnosis and treatment mediator in lung fibrosis. Inflammation. 2012;35:1747–1752. doi: 10.1007/s10753-012-9493-y. [DOI] [PubMed] [Google Scholar]
- Uzun H, Bitik O, Hekimoğlu R, Atilla P, Kaykçoğlu AU. Angiotensin-converting enzyme inhibitor enalapril reduces formation of hypertrophic scars in a rabbit ear wounding model. Plast Reconstr Surg. 2013;132:361e–371e. doi: 10.1097/PRS.0b013e31829acf0a. [DOI] [PubMed] [Google Scholar]
- Werner H, Le Roith D. New concepts in regulation and function of the insulin-like growth factors: implication for understanding normal growth and neoplasia. Cell Mol Life Sci. 2000;57:932–942. doi: 10.1007/PL00000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaron A, Naider F. Proline-dependent structural and biological properties of peptides and proteins. Crit Rev Biochem Mol Biol. 1993;28:31–81. doi: 10.3109/10409239309082572. [DOI] [PubMed] [Google Scholar]