Figure 3. The C-terminus of Rpn11 is capable of recruiting missing module 2 subunits.
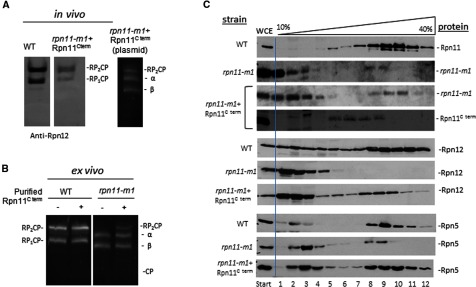
(A) The C-terminus fragment of Rpn11 was expressed as a separate gene product in rpn11–m1 background; proteasome species were compared with WT by in-gel peptidase activity (right) followed by immunoblotting by anti-Rpn12 (left). (B) WCE of rpn11–m1 (or WT) was incubated for 30 min with or without a recombinant polypeptide corresponding to the C-terminus of Rpn11; proteasomes were then visualized by in-gel peptidase activity. (C) WCE of WT, rpn11–m1 and rpn11–m1 expressing the C-terminal fragment were resolved by glycerol gradient; all fractions were immunoblotted by anti-Rpn11 to monitor distribution of Rpn11 or its fragments.
