Figure 6. Prying open the two domains of Rpn11 in the 26S proteasome.
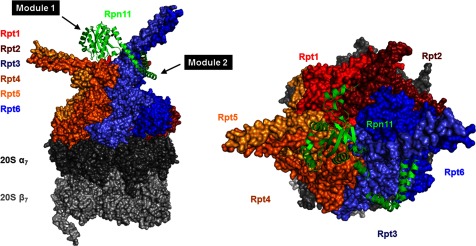
Position of Rpn11 (green) in 26S holoenzymes from EM model (PDB-4CR2) supports distinct interactions of MPN domain and C-terminal region. Lid PCI domains and Ub-processing factors in the base (Rpn1, Rpn2, Rpn10 and Rpn13) were rendered invisible in order to highlight the relative position of Rpn11 (green) to the ring of RPT ATPases. Rpt1 (red), Rpt2 (dark red), Rpt3 (light blue), Rpt4 (dark orange), Rpt5 (orange) and Rpt6 (blue). The hexameric RPT ring locks on to the 20S CP made up of the distal α-heptamer (dark grey) and the proteolytic β-heptamer (light grey). Note the pairing of RPTs via N-terminal coiled-coils: Rpt1–Rpt2, Rpt4–Rpt5 and Rpt3–Rpt6. Rpn11 catalytic MPN domain is situated directly above the centre of this hexameric RPT ring with its C-terminal segment pried away wrapping around the Rpt3–Rpt6 coiled-coil. The unique orientation of Rpn11 enables it to bridge between lid module 1 and module 2: remainder of module 1 subunits (Rpn5, Rpn6, Rpn8 and Rpn9) co-ordinate around the MPN domain of Rpn11, whereas the α-helix at its C-terminus participates in a helix bundle with C-termini of its paralogue Rpn8 and module 2 subunits (Rpn3, Rpn7 and Rpn12).
