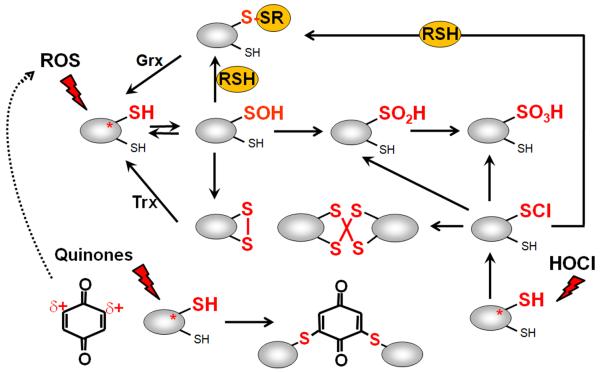
Figure 1. Thiol-chemistry of ROS, RES and HOCl with redox-sensing regulators.
Reversible thiol-oxidation by ROS leads first to a Cys sulfenic acid intermediate (R-SOH) that is unstable and reacts further to form intramolecular and intermolecular disulfides or mixed disulfides with LMW thiols, such as glutathione, bacillithiol, cysteine or CoASH, termed as S-thiolations. The Cys sulfenic acid can be also overoxidized to Cys sulfinic and sulfonic acids. Reactive electrophiles (RES) such as quinones have been shown to act via the S-alkylation and oxidation mode with quinone-sensing redox regulators. HOCl causes first chlorination of Cys thiol goups to the unstable sulfenylchloride which react further to form protein disulfides and S-thiolations in the presence of proximal thiols. In the absence of another thiol the sulfenylchloride rapidly forms irreversible Cys sulfinic or sulfonic acids (Hawkins et al, 2003).