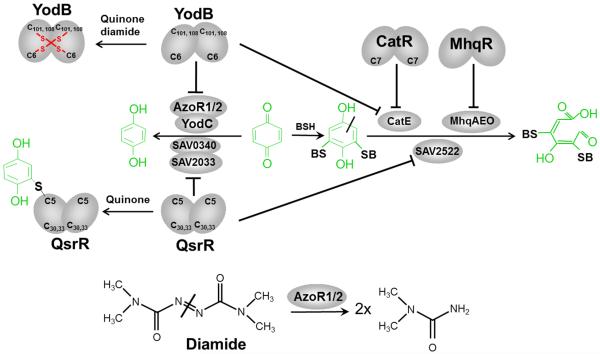
Figure 7. Redox-sensing of RES by the MarR/DUF24-regulators YodB in B. subtilis and QsrR in S. aureus.
Exposure of B. subtilis to quinones induces quinone detoxification regulons controlled by the MarR-type repressors MhqR, YodB and CatR. In S. aureus, the homologous quinone-sensing YodB (QsrR) repressor responds to quinones and controls paralogous quinone reductases, dioxygenases and nitroreductases. The redox-sensing YodB and CatR repressors are inactivated by the oxidative mode of quinone leading to disulfide formation that involves the conserved Cys6 or Cys7 residues (Antelmann et al, 2008; Antelmann & Helmann, 2011; Chi et al, 2010a; Chi et al, 2010b; Towe et al, 2007). In S. aureus, YodB (QsrR) with mutated C-terminal Cys residues senses quinones by thiol-S-alkylation of Cys5 leading to up-regulation of the dioxygenase SAV2522, the quinone reductase SAV0340 and the nitroreductase SAV2033 (Ji et al, 2013). The thiol-dependent dioxygenases MhqA, MhqE, MhqO, CatE of B. subtilis and SAV2522 of S. aureus are involved in specific ring-cleavage of quinones-S-adducts. The quinone reductases AzoR1, AzoR2 of B. subtilis and SAV0340 of S. aureus and the nitroreductases YodC, MhqN of B. subtilis and SAV2033 of S. aureus catalyze the reduction of quinones to redox stable hydroquinones.