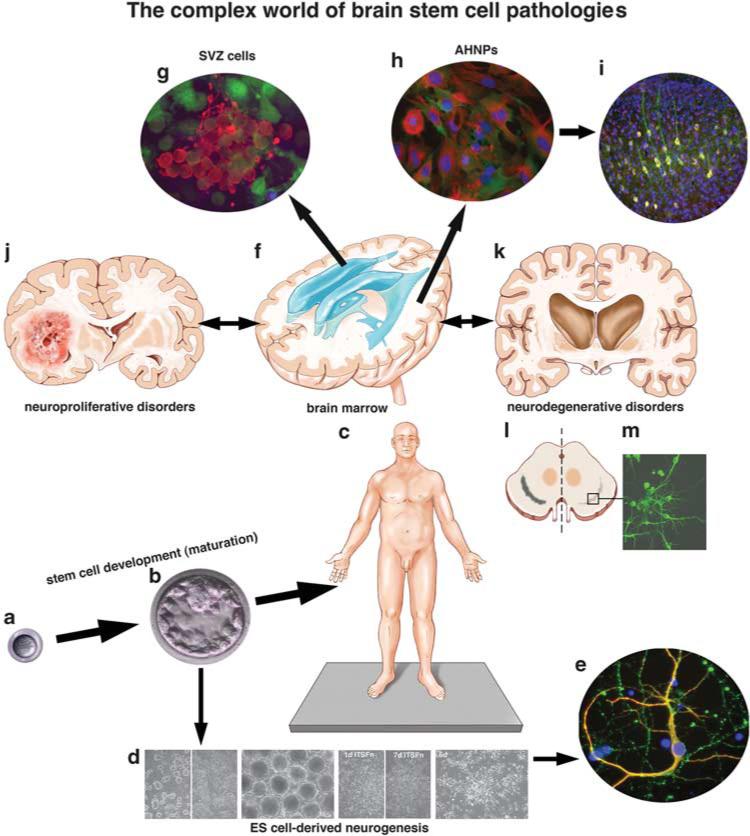
Figure 1.
The complex world of stem cell pathologies. Tissue-generating/repairing stem cells function from the beginning to the end of life (a–c): from the zygote (a), to the blastocyst (b), to a fully mature human being (c) where somatic or tissue-specific stem and progenitor cells reside in many, if not all, tissues and organs. Their lack or abundance may indicate stem cell pathologies. Although source and nature of most neurodegenerative and neuroproliferative disorders remain currently unknown, the study of stem cells in all stages of development may open new avenues for diagnosis and treatment of cancer and other diseases through clues from regenerative medicine. Embryonic stem cells (d) derived from the blastocyst inner cell mass (b) can give rise to all tissues and organs, including neural precursor cells that participate in embryonic and adult neurogenesis and generate different types of differentiated neurons (e) and glia. Neural stem and progenitor cells reside in the adult human brain in the periventricular subventricular zone (SVZ, and f) and the hippocampus, and participate in persistent neurogenesis (neuropoiesis,1 and g) that can be reactive cell genesis following neurodegenerative disease,5–8 injury or stroke.9 Progenitor cells can also be found in adult human brain cortical gray matter (so-called ‘Adult Human Neural Progenitor Cells’, or ‘AHNPs’, h, and see ref. 37) that can be massively propagated in vitro and seemingly amenable to reparative attempts in vivo, as grafting these cells into the cerebrum of adult SCID mice results in the generation of differentiated cells, including neurons (i). Neuropoiesis in the human brain can also lead to too much growth (j), as seen in our originally described cancer stem cell population involved in gliomagenesis.38 Attempted but failed regeneration of neurons from stem and progenitor cell populations in neurodegenerative diseases including Huntington's disease (k) suggests that reactive neurogenesis could be enhanced and exploited with molecular factors and drugs discovered from new bioassays and high-throughput screening approaches. This is exemplified in vitro from pilot studies in which we isolate cells from postmortem specimens of the substantia nigra in another neurodegenerative disease, Parkinson's disease (PD; l), and even though stem/progenitor cells in this nucleus do not successfully thwart the loss of dopaminergic neurons in this disease in vivo, they may be amenable to the generation of neurons (eg, beta III tubulin-positive) and dopamine-like neurons (tyrosine hydroxylase-positive) under the proper growth conditions in vitro (m), and then used for in vitro bioassaying for drug screening, or compared with cells generated from new reprogramming24,25 methods (ie, induced pluripotent stem cells, (iPSCs), as described for PD and other neurodegenerative diseases39,40). Ex vivo study and manipulation of these neural precursor cells may provide insights into tapping these cells for self-repair in PD and other neurodegenerative diseases. All of these examples illustrate the potential roles for stem cells in the etiology of disease (eg, neurodegenerative or neoplastic), as well as during attempted repair and regeneration of tissues and organs following injury or disease. The neostriatum atrophies significantly through progressive stages of HD (k), with loss of both neurons and glia over time;41 it is clear that reactive neurogenesis with stem cell enhanced proliferation occurs at the expense of neuronal progenitor cells, however,42 that therefore fails to hold the basal ganglia and cortical cell loss at bay without a necessary compensatory reactive neurogenesis. Figures ‘d and e’ are from the study by Goetz et al (PNAS 2006; 103:11063–8); Figure ‘g’ is from the study by Scheffler et al (PNAS 2005; 102:9353–8) and, Figures ‘h and i’ are from the study by Walton et al.37