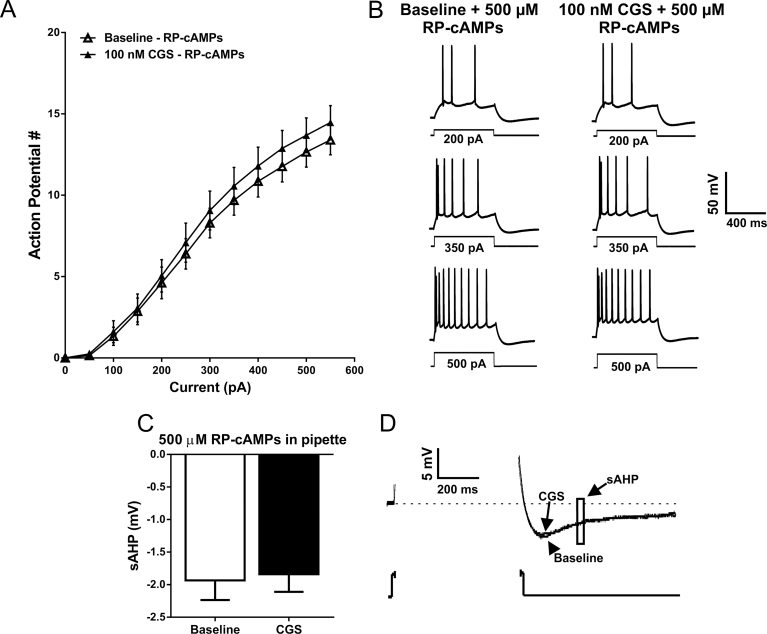
Figure 8.
Inclusion of a protein kinase A (PKA) inhibitor in the patch pipette blocks the CGS-associated increase in basolateral amygdala (BLA) pyramidal cell intrinsic excitability and the reduction in slow afterhyperpolarization (sAHP) amplitude. (A) The relationship between depolarizing current steps and the firing rate of action potentials elicited at baseline with the inclusion of the PKA inhibitor RP-cAMPs included in the patch pipette (open triangles) and with RP-cAMPs in the pipette following bath application of CGS-21680 (closed triangles) (NS, F CGS (1, 8)=2.951, P=.1241; F interaction (11, 88)=1.702 P=.0859, 2-way RM ANOVA; n=9). (B) Representative voltage responses to depolarizing current steps recorded from BLA pyramidal neurons with RP-cAMPs included in the patch pipette at baseline and following the bath application of 100nM CGS-21680. (C) Bar graph indicating that the CGS-associated reduction in sAHP amplitude is block when the protein kinase A (PKA) inhibitor is included in the patch pipette (NS, P=.2287, paired t test; n=9). (D) Representative voltage responses to a depolarizing current step illustrating that when RP-cAMPs is included in the patch pipette, CGS-21680 no longer modulates the amplitude of the sAHP.