Abstract
Background
Until recently, investigations of the normal patterns of motility of the healthy human colon have been limited by the resolution of in vivo recording techniques.
Methods
We have used a new, high-resolution fiber-optic manometry system (72 sensors at 1-cm intervals) to record motor activity from colon in 10 healthy human subjects.
Key Results
In the fasted colon, on the basis of rate and extent of propagation, four types of propagating motor pattern could be identified: (i) cyclic motor patterns (at 2–6/min); (ii) short single motor patterns; (iii) long single motor patterns; and (iv) occasional retrograde, slow motor patterns. For the most part, the cyclic and short single motor patterns propagated in a retrograde direction. Following a 700 kCal meal, a fifth motor pattern appeared; high-amplitude propagating sequences (HAPS) and there was large increase in retrograde cyclic motor patterns (5.6±5.4/2 h vs 34.7±19.8/2 h; p < 0.001). The duration and amplitude of individual pressure events were significantly correlated. Discriminant and multivariate analysis of duration, gradient, and amplitude of the pressure events that made up propagating motor patterns distinguished clearly two types of pressure events: those belonging to HAPS and those belonging to all other propagating motor patterns.
Conclusions & Inferences
This work provides the first comprehensive description of colonic motor patterns recorded by high-resolution manometry and demonstrates an abundance of retrograde propagating motor patterns. The propagating motor patterns appear to be generated by two independent sources, potentially indicating their neurogenic or myogenic origin.
Keywords: colonic physiology, high-resolution manometry, myogenic, neurogenic, peristalsis
1. INTRODUCTION
Despite its size and physiological importance, the motor behavior of the human colon remains poorly understood. Many disorders are associated with abnormalities in colonic motility, yet investigation of normal human colonic motor function, including specific changes in disease states, is still quite rudimentary. Propulsion and mixing of colonic content are the result of coordinated contractions and relaxations of the circular and longitudinal smooth muscle layers, which are controlled by a combination of myogenic and neurogenic mechanisms. In many animal species, ranges of neurogenic and myogenic motor patterns have been described.1–6 In contrast, little is known about the in vivo nature of motor patterns of human colonic motility.
The lack of understanding is primarily due to methodological constraints. Early observational studies of transit in the human colon, using X-rays, described propulsive motility patterns in reasonable detail.7–10 However, when the dangers of radiation exposure became known, less precise techniques had to be substituted. The best established method is colonic manometry, which measures changes in intraluminal pressure caused by contractions of the smooth muscle layers. However, in the past, manometric devices were limited to a maximum of about 16 recording sites.11 As the length of the adult colon is 0.8–1.3 m (depending on recording conditions), manometric recording sensors have been typically spaced at intervals of 7 cm or more, to record from the entire length of the organ.12–18 This type of low-resolution manometry has reliably identified one very distinctive pattern of colonic motility: ‘high-amplitude propagating sequences’ (HAPS) that occasionally traverse long sections of the colon. Other activity detected by low resolution manometry has proved difficult to classify and has usually been grouped together under collective headings of ‘segmental activity’ or ‘non-propagating activity’.19
A high-resolution manometry catheter, based on Fiber Bragg gratings, has recently been developed.20 It has up to 72 pressure sensors spaced at 1-cm intervals, providing considerable improvements in resolution. The accuracy of this type of catheter to record motor patterns has been validated in specimens of small and large bowel of laboratory animals, in vitro21,22 and in clinical studies of human colon.23,24 When channels from high-resolution recordings are deleted, until the resolution mimics that of low-resolution catheters, it has been shown that sensor spacing above 2 cm can lead to significant misclassification of many small propagating motor patterns.25 Furthermore, high-resolution recording from the colon of normal human subjects clearly demonstrates that much of the activity that has been previously labeled as ‘non-propagating’ actually consists of propagating motor patterns that travel short distances along the colon.25
The present study used high-resolution, fiber-optic manometry to investigate the full complement of motor patterns in the normal human colon and then defined the changes in these motor patterns following ingestion of a meal. In addition, on the basis of characteristic of the recorded pressure events, we provide mechanistic insight into the generation of the identified motor patterns.
2. METHODS
Healthy control subjects
Colonic manometry was performed in 10 healthy human controls (7 women; median age 53 years; range 27–69 years). Seven of these studies were performed at Flinders Medical Centre, in South Australia, and three at St. George Hospital in Sydney, New South Wales. All subjects had a normal bowel habit, defined as between three bowel movements a day and one bowel movement every 3 days, with no gastrointestinal symptoms. None had a history of metabolic, neurogenic, or endocrine disorders, and none was taking regular medication or had prior major gastrointestinal surgery. None of the women was pregnant.
In addition to these 10 healthy controls, pilot studies in four healthy subjects (aged 22–48) were used to develop criteria to distinguish motor patterns from visual inspection of traces (see Discriminant, logistic, and cluster analysis). The pilot studies ran for a minimum of 24 h and used several protocols, and were also used to test the durability of the fiber-optic manometry catheters and the reliability of the software to capture, display, and analyze manometric data.
All participants gave written, informed consent and the studies were approved by the Human Ethics Committees of the South Eastern Area Health Service, Sydney (05/122; May 2010), and The Southern Adelaide Health Service/Flinders University Human Research Ethics Committee (419.10; March 2011).
Fiber-optic catheter
At both hospitals colonic intraluminal pressure was recorded with a high-resolution fiber-optic catheter containing 72 sensors spaced at 1-cm intervals (Fig. 1).20,23 The catheter was attached to a spectral interrogator unit (FBG-scan 804; FOS&S, Geel, Belgium) and pressures were recorded using a customwritten LabVIEW program (National Instruments, Austin, TX, USA). Analysis of the fiber-optic data was performed using software (which we called ‘PlotHRM’) developed by one of the authors (LW). PlotHRM was written in Matlab (MathWorks, Natick, MA, USA) and JavaTM (Sun Microsystems, Santa Clara, CA, USA).
Figure 1.
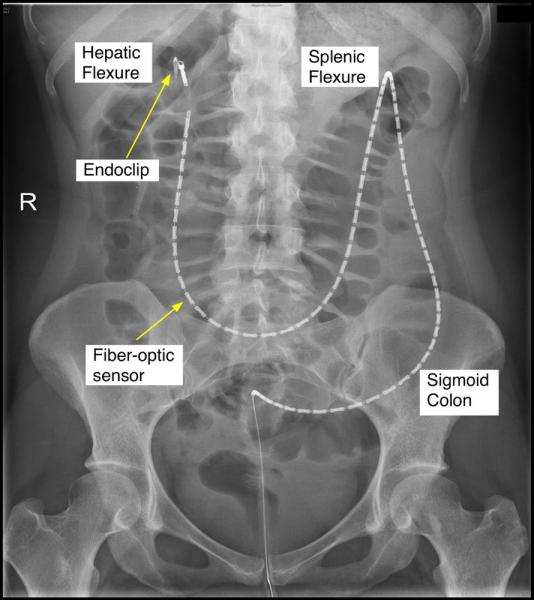
X-ray image of the fiber-optic catheter positioned in the healthy human colon. The tip of the catheter can be seen at the hepatic flexure. The middle of each white segment is the position of each pressure sensor.
Colonoscopic placement of the fiber-optic catheter
The technique to place colonic catheters has been described in detail previously.26 At both hospitals, on the day prior to the procedure, the bowel was cleared using sodium picosulphate and polyethylene glycol (Pharmatel Fresenius Kabi Pty Ltd, Hornsby, NSW, Australia). Patients drank only clear fluids overnight. Lying in the left lateral position, with conscious sedation using midazolam and fentanyl, the manometry catheter was introduced with a colonoscope and clipped to the mucosa of the ascending or proximal transverse colon using Endoclips (Resolution Clip, Boston Scientific, MA, USA)26 (Fig. 1).
Study protocol
Subjects were taken to the general ward and recordings were commenced within 60 min of the subject waking. After a 2-h basal recording period, all subjects were given a 700 kCal meal (24% protein, 43% fat, 33% carbohydrate). The meal consisted of 300 mL of TwoCal HN Vanilla (Abbott Nutrition, Columbus, OH, USA) and a chicken sandwich. Colonic pressures were then recorded for a further 2 h, after which an abdominal X-ray was performed so that sensor placement could be determined (Fig. 1). For the purpose of analysis, sensors were localized to the proximal colon region (ascending & transverse colon), the descending colon region, or the sigmoid colon region.
Analysis of manometric data
In each manometric recording, obvious artifacts of simultaneous pressure events that spanned all recording channels were digitally removed as previously described.27
The manometric traces consist of a variety of ‘pressure events’ (individual phasic pressure excursions recorded by a sensor). Two categories of rapid small amplitude pressure events were commonly identified. The first occurred at a very high frequency (60–80/min) and probably reflected cardiovascular pulse. The second consisted of pressure events occurring commonly at 16–20/min. These were mostly recorded synchronously across multiple channels. Because of their frequency and their synchronicity, they were deemed likely to have been caused by respiratory movements. These two types of pressure events did not form part of any further analysis.
Visual identification of propagating pressure events
When viewed across multiple channels, pressure events often formed recognizable propagating motor patterns. Propagation was confirmed if a pressure event peak occurred in four or more adjacent channels (i.e., ≥4 cm), each with a trough-to-peak amplitude of at least 5 mmHg. If a pressure event returned to baseline before the pressure event in the adjacent channels started, then the two events were not considered part of the same propagating motor pattern. Propagating motor patterns were classified on the basis of whether they occurred cyclically or as single events, whether their propagation was anterograde (anally propagating), retrograde (orally propagating), by their propagation velocity and by the distance over which they traveled. A full description of each type of propagating motor pattern can be found in the Results section.
Comparisons of characteristics (numbers, amplitude, velocity, extent of propagation) for each type of propagating motor pattern before and after the meal were compared with a non-parametric Wilcoxon matched-pairs signed rank test. Comparisons between different kinds of propagating motor patterns were conducted using a Mann–Whitney test (GraphPad Software, Inc., La Jolla, CA, USA).
Measuring and plotting durations and amplitudes of individual pressure events
To compare pressure events that comprised different motor patterns, their duration was plotted against amplitude. To achieve unbiased samples, software in the PlotHRM placed a random mark on a random channel and the observer measured the next appropriate pressure event to the right of the mark.
Thirty examples of pressure events from each type of motor pattern (see Results) were analyzed for each of the control subjects.
Discriminant, logistic, and cluster analysis of the shapes of pressure events belonging to different motor patterns
To establish whether complex features of pressure events differed systematically between different propagating motor patterns, we performed several statistical analyses. Each clearly defined pressure event within an identified motor pattern was selected and automatically measured for the following characteristics, using routines in the PlotHRM software25;
Maximal amplitude (trough to peak);
Maximum gradient of the rising phase;
Maximum gradient of the falling phase;
latency from start to half amplitude of the rising phase;
latency from start to half amplitude of the falling phase;
In some instances the first or last pressure events within a motor pattern overlapped with other motor patterns. These pressure events were excluded from this analysis.
In total, 8597 pressure events were analyzed in IBM SPSS v19 (IBM Corporation, Armonk, NY, USA). Because of the relative scarcity of HAPS (see Results) in the meal response protocol, the characteristics of pressure events from HAPS identified in the pilot data were also included. Measures of pressure event asymmetry were derived from the five variables above, including the ratio of the rising and falling half maximum widths, and the ratio of the rising and falling gradients. Each pressure event was additionally classified by subject, by the type of propagating motor pattern that it came from (visual identification; see Results), and by the anatomical region of the colon in which it occurred. Initial exploratory data analyses used multivariate analysis of variance (MANOVA; homogeneous subsets of data, identified post hoc with Ryan-Einot-Gabriel Welsch F [REGWF] tests) and factor analysis, with principal component extraction with varimax rotation. Data classification methods included cluster analysis (Two-Step procedure using log-likelihood measures of distance), discriminant analysis (all variables entered simultaneously), and logistic regression with all variables entered either simultaneously or with forward selection using maximum-likelihood ratio criteria.28–31 Relative frequencies of cases according to different classifications were compared with contingency tables and chi-squared tests. In general, significance levels for hypothesis testing were set at p < 0.05 and parameter estimates were estimated using 95% confidence limits.
Measurement of average pressure
As a general measure of total contractile activity, irrespective of the specific motor patterns, we calculated the mean pressure for each of the three colonic regions on the basis of the sensor location (see above). HAPS were removed before calculating average pressures. For each colonic region (proximal, descending, and sigmoid), 12 × 10-min epochs were selected either side of the meal, and pressure at every point, across all channels, was summed and divided by the number of samples (data acquired at 10 Hz).
A mixed ANOVA (IBM SPSS v19) was conducted to assess whether there were differences according to colonic region or at different points in the recording period.
3. RESULTS
3.1 Description of propagating motor patterns
High-amplitude propagating sequences
Consistent with our previous studies, these propagating motor patterns consisted of an array of pressure events with the majority having a trough-to-peak amplitude of >116 mmHg (Fig. 2A).32 The pressure events in HAPSs had a duration of 23.2 ± 4.9 s and a peak amplitude of 241.8 ± 123.4 mmHg. The average extent of propagation was 33 ± 12 cm (range 11–50 cm) with a mean propagation velocity of 0.4 ± 0.1 cm/s, and they always progressed in an antegrade direction. In all instances, these events started in the proximal colon. These motor patterns made up 1.4% of all propagating motor patterns detected.
Figure 2.
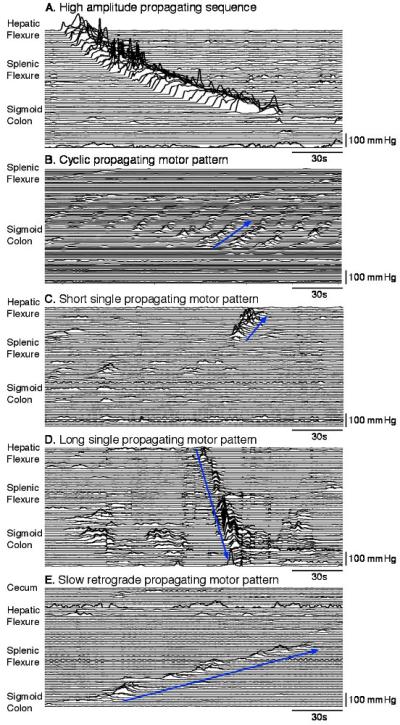
Examples of the five main types of propagating motor pattern identified by visual inspection of multi-channel manometric traces. (A) High-amplitude propagating sequence; (B) cyclic propagating motor pattern, in this instance propagating in an retrograde (oral) direction (blue arrow); (C) short single propagating motor pattern – in this case moving in a retrograde direction (blue arrow); (D) long single propagating motor pattern – all of these moved in an antegrade (anal) direction (blue arrow). (E) Slow retrograde propagating motor pattern (blue arrow), which was only observed in two subjects, and only during the fasted state.
Cyclic motor patterns
Repetitive propagating pressure events with cyclic frequency of 2–6/min occurred in all subjects (Table 1; Fig. 2B). Overall these were the most frequent motor patterns recorded. They were made up of pressure events with an average duration of 12.6 ± 2.9 s, and amplitude of 23.1 ± 21.4 mmHg, these motor patterns propagated in a retrograde (retrograde cyclic motor pattern) or antegrade (antegrade cyclic motor pattern) pattern, or occasionally were aligned synchronously across several sensors.
Table 1.
The propagating motor patterns identified in each of the subject prior to and after the meal.
| Subject | Pre-Meal | Post-Meal | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HAPS | Cyclic | Short single | Long, single | Slow retrograde | HAPS | Cyclic | Short single | Long, single | Slow retrograde | |
| 1 | - | - | ✓ | ✓ | - | ✓ | ✓ | ✓ | ✓ | - |
| 2 | - | ✓ | ✓ | - | - | - | ✓ | ✓ | ✓ | - |
| 3 | - | ✓ | ✓ | - | - | ✓ | ✓ | ✓ | - | - |
| 4 | - | - | ✓ | ✓ | - | - | ✓ | ✓ | ✓ | - |
| 5 | - | - | ✓ | - | - | ✓ | ✓ | ✓ | ✓ | - |
| 6 | - | ✓ | ✓ | - | ✓ | ✓ | ✓ | ✓ | - | - |
| 7 | - | ✓ | ✓ | - | - | - | ✓ | ✓ | - | - |
| 8 | - | - | ✓ | - | - | - | ✓ | ✓ | ✓ | - |
| 9 | - | - | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | - |
| 10 | - | ✓ | ✓ | ✓ | - | - | ✓ | ✓ | ✓ | - |
HAPS: High amplitude propagating sequence
Cyclic: propagating motor pattern
Short single: propagating motor pattern
Long single: propagating motor pattern
Slow Retrograde: propagating pattern
Retrograde and anterograde cyclic propagating motor patterns had similar amplitudes, waveforms, velocities of propagation, extents of propagation, and durations of contraction (see Table 2). The synchronous propagating motor patterns also had similar characteristics (data not shown). Cyclic motor patterns made up 69.1% of all propagating activity and while they were observed in the proximal and descending colon, the majority (76%) were identified in the sigmoid colon.
Table 2.
Characteristics of propagating motor patterns before and after a meal. The well characterized high amplitude propagating sequences were not included in this table.
| Pre-Meal | Post – meal | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cyclic | Short Single | Long Single | Cyclic | Short Single | Long Single | |||||
| Antegrade | Retrograde | Antegrade | Retrograde | Antegrade | Retrograde | Antegrade | Retrograde | |||
| Number/2hr | 6.6 ± 11.4 | 5.6 ± 5.4 | 4.3 ± 3.7 | 9.0 ± 7.3 | 3.0 ± 1.4 | 7.5 ± 2.1 | 34.7 ± 19.8* | 2.3 ± 1.9 | 7.0 ± 4.2 | 2.6 ± 1.7 |
| Velocity (cm/s) | 1.0 ± 1.3 | 0.7 ± 0.6 | 0.6 ± 0.4 | 0.3 ± 0.1 | 1.8 ± 1.2 | 0.8 ± 0.3 | 0.9 ± 0.4 | 0.5 ± 0.3 | 0.6 ± 0.3 | 2.0 ± 0.8 |
| Extent of propagation (cm) | 4.3 ± 0.8 | 4.8 ± 1.4 | 6.2 ± 2.8 | 6.0 ± 2.1 | 40.8 ± 8.4 | 6.6 ± 2.0 | 8.1 ± 2.6** | 8.4 ± 1.9 | 8.3 ± 2.0 | 44.4 ± 13.7 |
| Amplitude (mmHg) | 27.9 ± 11 | 40.2 ± 22.2 | 50.2 ± 24.7 | 35.6 ± 14.6 | 43.8 ± 12.7 | 51.5 ± 13.6 | 48.6 ± 19.9 | 56.4 ± 44 | 39.9 ± 10.8 | 52.3 ± 17.9 |
| Duration of pressure waves (s) | 10.9 ± 1.3 | 10.6 ± 1.4 | 14.3 ± 4.7 | 14.1 ± 4.8 | 9.3 ± 1.3 | 11.4 ± 1.6 | 10.2 ± 1.7 | 13.5 ± 4.2 | 11.9 ± 2.0 | 9.8 ± 1.3 |
p < 0.001 (significant increase after the meal)
p = 0.03 (significant increase after the meal)
Short single motor patterns
This pattern occurred in isolation separated from other propagating motor patterns by intervals of more than 1 min (Fig. 2C). They were observed in all subjects (Table 1). The component pressure events had a mean duration of 15.6 ± 4.1 s (range: 7–32 s) and an amplitude of 58.1 ± 26.7 mmHg. Retrograde short single motor patterns and anterograde short single motor patterns had a similar extent of propagation (~7 cm see Table 2) and they traveled at a velocity of 0.5 ± 0.3 cm/s (Table 2). These motor patterns originated primarily in the proximal (42%) or sigmoid colon (43%) and they made up 24.2% of all propagating motor patterns.
Long single motor pattern
These occurred as single pressure events, which propagated over long distances (Fig. 2D). These motor patterns were always separated by intervals of more than 1 min, when they occurred repetitively. Long single motor patterns were only recorded in 7 of 10 subjects (Table 1). Most originated in the proximal colon (76%) with the remaining 24% originating in proximal descending colon. All propagated in an antegrade direction. They were never seen to migrate in a retrograde direction. They were distinguished from short single motor patterns because they propagated over significantly longer distances along the colon (40.8 ± 8.4 cm; p < 0.0001; Table 2), often reaching the distal descending or sigmoid colon. They also traveled at a significantly greater velocity (1.8 ± 1.2 cm/s; p < 0.0001).
A rare but very distinctive propagating motor pattern, observed in just two subjects, was the retrograde slowly propagating motor pattern (Fig. 2E; Table 1). They traveled at less than 0.5 cm/s over distances of more than 40 cm, starting in the sigmoid colon and extending into the transverse colon and made up only 0.3% of all propagating motor patterns. These were analyzed further.
3.2 Classification of pressure events according to amplitude and duration
Plotting the duration against the amplitude (Fig. 3) shows a tendency for larger pressure events to be associated with longer durations (across all plotted events r = 0.261, df = 394, p < 0.01). However, there is significant overlap between the pressure events that make up each of the propagating motor patterns (Fig. 3). Therefore, such a simplistic correlation is not sufficient to classify individual pressure events as belonging to specific propagating motor patterns.
Figure 3.
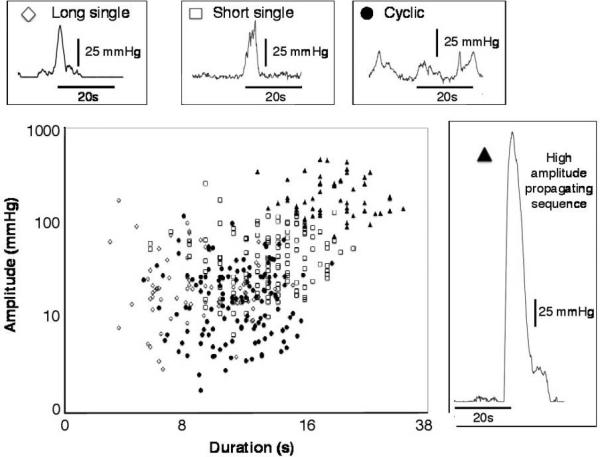
The relationship between duration and amplitude of the component pressure events of the four main propagating motor patterns. The long single (open diamonds), short single (open squares), cyclic (closed circle), and high amplitude (closed triangle) motor patterns do not form clearly defined clusters; there is considerable overlap between the groups. Note that both the x- and y-axis use logarithmic scales.
Discriminant, logistic, and cluster analysis of the shapes of pressure events belonging to different motor patterns
In comparison of the shape (duration, gradient, and amplitude) of pressure events that made up the four main types of motor pattern (see Fig. 2A–D), the initial MANOVA showed that visually identified pressure events that formed HAPS differed from the pressure events that made up the other three propagating motor patterns (Fig. 4A) in all measured parameters (overall, F(15,25773) = 803 (Wilks lamba); p < 0001). Thus, the pressure events in HAPS had significantly larger (three- to fivefold) maximum amplitudes, left and right half-maximum widths, and left and right mean gradients compared with other pressure events in the other motor patterns. There were statistically significant differences (REGWF tests, p < 0.05) in some parameters between pressure events of long, short, and cyclic motor patterns, but these were considerably smaller (0.1- to 0.5-fold). The differences between pressure events in HAPS and in the other motor patterns were preserved when the MANOVA model allowed for subject and sequence variances. Factor analysis based on pressure event properties identified two significant principal components: one had strong correlations with maximum amplitude and gradient (55% of variance) and the other with the duration of the pressure event (28% of variance). Thus, HAPS could be reliably distinguished from the three other major patterns, which were more similar to one another than to HAPS.
Figure 4.
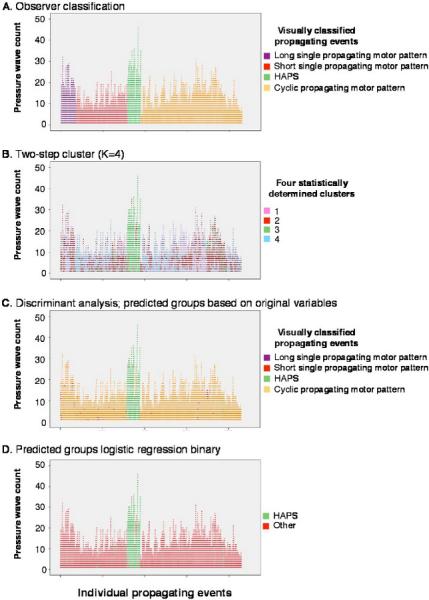
Statistical analysis of the pressure events that made up the four main propagating motor patterns. Each vertical series of points represents an individual propagating motor pattern starting at the x-axis (A) The full set of propagating motor patterns used in this analysis are color-coded according to the classification scheme developed in this study. (B) Shows the same set of pressure events, in the same order, but color-coded according to their cluster analysis group (Two-Step cluster, k = 4 groups). Note that cluster 3 (green points) closely matches pressure events from high amplitude propagating sequences (HAPS) shown in (A). The other three clusters do not map onto pressure events from the other three motor patterns. (C) The same set of pressure events identified according to discriminant analysis. HAPS pressure events are well discriminated from all other events (compare with the green points in A). The analysis could not reliably distinguish the other three motor patterns on the basis of their component pressure waves. (D) Logistic regression again clearly distinguished HAPS pressure events (green points, compare with A) from all other, which could not be reliably subclassified.
Cluster analysis, based on pressure event properties, consistently grouped those that formed HAPS into largely homogeneous sets. When the number of clusters was preset at k = 4 (to represent the four visually identified classes of propagating motor patterns), one cluster contained 84% of visually classified HAPS pressure events in the total data set (Fig. 4B). Changing the number of clusters from 2 to 6 made little difference in the grouping of HAPS pressure events. Consistent with the factor analysis, the major variable contributing to the clustering was the pressure event maximum amplitude, followed by the average right gradient of the pressure event.
Given the ability of cluster analysis to distinguish HAPS from the three remaining motor patterns, discriminant analysis was then used to test how well pressure events could be assigned to the four visually discriminated motor patterns. The analysis generated three canonical discriminant functions. The dominant function, explaining 97% of the variance, was most highly correlated with maximum amplitude (r = 0.70) of pressure events; the remaining functions were correlated with the gradient (r = 0.78) and width (r = 0.63), respectively, of the pressure events. Based primarily on the first discriminant function, the analysis correctly identified 84% of pressure events as belonging to the visually classified HAPS group. Consistent with the small explanatory power of the second and third discriminant functions, the identification of the remaining pressure events was not well matched to the visually classified motor patterns. Indeed, they were mostly predicted to form one large group (Fig. 4C). When the analysis was repeated, forcing a two-group classification (visually classified HAPS vs the rest) using either discriminant analysis or logistic regression, a similar proportion (around 84%) of HAPS pressure events were correctly identified (Fig. 4D). Controlling for potential subject or the effects of a sequence of pressure events did not change the classification rate.
Taken together, these analyses showed that visually identified HAPS form a natural group that could be separated from all remain propagating motor patterns, in an unbiased manner, simply on the basis of the characteristics of their component pressure events. The remaining three main propagating motor patterns can only be visually distinguished on the basis of their propagation direction, propagation extent, and propagation velocity.
3.3 The effect of a meal on colonic motor activity
Preprandial motor patterns
The average pressure, measured across 10-min epochs, was quite constant over a period of 2 h prior to the meal (Fig. 5A). Average pressure did not vary consistently between the three regions studied (proximal, descending, or sigmoid colon; Fig. 5A). The motor activity that was observed prior to the meal consisted mainly of non-propagating pressure events. No HAPS (Table 1) were recorded during the fasted period, and the other major motor patterns (Fig. 2B–D) occurred sporadically (Table 1) and in low numbers (Table 2 and Fig. 5B). The speed and extent of propagation, amplitude and duration of pressure events of each propagating motor pattern are summarized in Table 2.
Figure 5.
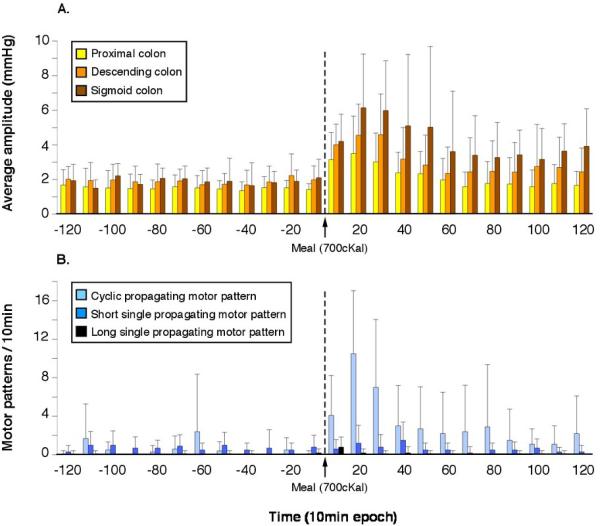
(A) the average pressure in three colonic regions averaged in 10-min bins, after high-amplitude propagating sequences had been manually removed. Prior to the meal average pressure showed little fluctuation and there were no significant differences between the three regions (proximal, descending, and sigmoid colon). After the meal, there was a significant (p < 0.03) increase in average pressure in all three regions, which peaked within the first 20 min after starting to eat. (B) The number of propagating motor patterns per 10-min interval before and after a meal. Note that the number of cyclic propagating motor patterns increased markedly after the start of the meal (p < 0.0001), but there were no significant effects on other propagating motor patterns. Of the cyclic motor patterns, retrograde cyclic patterns accounted for ~97% of the increase caused by the meal.
Postprandial motor patterns
After the meal, there was a significant increase (p < 0.03) in motor patterns in all regions of the colon. This increase was rapid, occurring within minutes of starting the meal (Figs 5A and 6). In the proximal and descending colon, this increase was evident for ~30 min before returning to baseline (Fig. 5A). In the sigmoid colon, motor activity remained elevated for at least 2 h postprandially (Figs 5A and 6).
Figure 6.
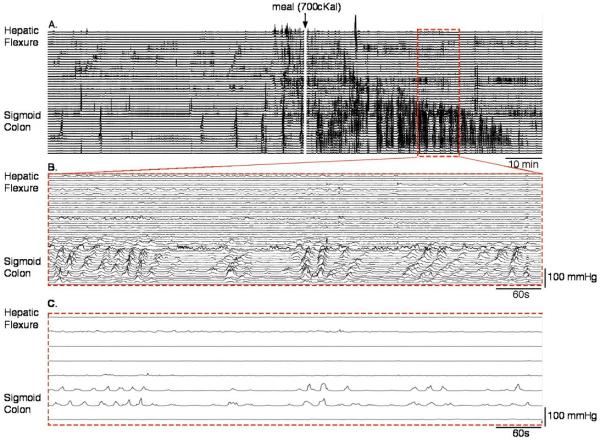
(A) A condensed manometric recording of the colon of a single subject over a 2-h period (1 h before and after a meal). The white line in the middle of the trace shows where the subject started the meal. Note the rapid increase in number of pressure events after the meal. (B) It is an enlargement of the red hatched box in A. Numerous retrograde cyclic propagating motor patterns are visible; they clearly comprise majority of the increased contractile activity induced by the meal. (C) It shows the same data as seen in B, but shown as a traditional low-resolution trace (lines spaced at 7 cm). All propagation is lost and these data would have been labeled as ‘non-propagating’.
High-amplitude propagating sequences appeared in 5 of 10 subjects after the meal (Table 1). In all cases, these sequences were identified 15–70 min after the start of eating. In one male subject, four HAPS were recorded in a 20-min period after eating. In another male subject, three HAPS were observed within a 20-min period. Three female subjects had one HAPS each.
Apart from HAPS, the major effect on colonic motility of ingesting a meal was a large increase in cyclic retrograde propagating motor patterns, which increased from 5.6 ± 5.4/2 h to 34.7 ± 19.8/2 h (p < 0.001; Fig. 5B). This was particularly marked in the sigmoid colon (Fig. 6). These retrograde cyclic propagating motor patterns tended to traverse a greater distance along the colon after the meal than before (4.8 ± 1.4 cm before vs 8.1 ± 2.6 cm after the meal; p = 0.03). Antegrade cyclic propagating motor patterns showed no change after the meal, with retrograde cyclic patterns accounting for 97% of the increase in cyclic patterns after eating (Table 2). There was also no significant increase in either short or long single propagating motor patterns (Table 2; Fig. 5B).
4. DISCUSSION
In this study, we have described the motor patterns of the normal human colon both pre- and postprandially, using the new technique of high-resolution fiber-optic intraluminal manometry. While we have recorded HAPS, reported by many previous workers in this field, the significance of our finding lies in the ability to discriminate a number of smaller amplitude motor patterns that had hitherto been clustered together under the heading of ‘segmental’ or ‘non-propagating’ activity. High-resolution recordings have revealed that many of these low-amplitude motor patterns actually propagate along the colon and that the proportion of retrogradely propagating motor patterns has been greatly underestimated in previous colonic manometry studies.17,18,33–36
Origin of HAPS
In 1907, using X-rays after a bismuth meal, Arthur Hertz measured transit through the human digestive tract and described the occasional events where large amounts of digesta moved rapidly from one section of the colon to an aboral section.37 These ‘mass movements’ later identified and named ‘high-amplitude propagating contractions’15 or ‘HAPS’32 in manometric studies. In animal studies, similar motor patterns can be evoked by intraluminal stimuli and have been termed ‘peristaltic contractions’, ‘giant migrating contractions’, or ‘mass peristaltic events’. They have been shown to be propulsive and are dependent on enteric neural circuits.1–3,5,6,38,39
In our current study, HAPS were only observed in 5 healthy subjects and made up only 1.4% of all identified propagating motor patterns. Their relative scarcity may have been influenced by the study protocol. Manometric recording started within an hour of catheter placement, in a prepared (empty) colon, and stopped 4 h later. There was little opportunity for colonic filling. A similar protocol used by De Schryver et al.40 identified postprandial HAPS in only 4 of 10 healthy controls. In contrast, our previous colonic manometry studies have been performed either in an unprepared colon18,32,41 or 1 day after catheter placement.36,42 In those studies, HAPS were considerably more abundant. It is likely that large volumes of colonic content facilitate activity in the enteric neuronal pathways that underlie HAPS. For example, undigested starch in the colon has been shown to increase the incidence of these motor patterns.43
High-amplitude propagating sequences are not only activated by luminal distension; they can also be triggered by intraluminal chemical stimuli (including bisacodyl, chenodeoxycholic acid, and short-chain fatty acids).14,18,44,45 Lidocaine applied to the colonic mucosa prevents the excitatory action of these chemical stimuli, but does not block progression of HAPS.12 It is likely that chemical stimuli act by activating mucosal enteric afferent neurons, which then initiate self-sustaining HAPS. Taken together, these findings suggest that HAPS can be activated by mechanical distension or by chemical stimuli acting on underlying enteric circuits. The fact that they can appear rapidly after a meal16,18,36 also suggests that the enteric circuits can be positively modulated by extrinsic neural inputs, after feeding.
Colonic myogenic activity
While HAPS could be labeled as being neurogenic in origin, myogenic activity is also well established to occur in the distal digestive tract, driven by pacemaker networks of interstitial cells of Cajal (ICC).46 In human and dog colon, cyclic slow waves at 2–4/min are generated by ICC located in the circular muscle layer, giving rise to contractions with this characteristic frequency range.47 Electrical activity with similar frequency has been recorded by electromyography in the human sigmoid colon (‘rhythmic stationary bursts’).48 Slow wave activity has also been temporally associated with small amplitude variations of intraluminal pressure.49 In the present study, all the healthy subjects had regular low-amplitude rhythmic phasic pressure waves at ~3/min frequency that propagated mainly in a retrograde direction (‘cyclic motor patterns’). It is very likely that these propagating motor patterns therefore represent the physiological consequences of slow waves in the human colon.50–55
Myogenic cyclic motor pattern in response to the meal
Many previously, low-resolution multi-channel recordings from human colon identified that ingestion of a meal increases colonic motor activity.18,56–62 Within a few minutes of starting a meal, we detected a large increase in the number of cyclic propagating motor patterns, particularly those that appear to originate in the sigmoid colon (see Fig. 2B). It is very striking that the increase is solely due to retrograde cyclic motor patterns without a concomitant increase in anterograde cyclic motor patterns, in the same region. We suggest that this is due to the appearance of a frequency gradient of slow waves, with a dominant highfrequency area in the distal sigmoid colon, with consequent retrograde propagation. This explanation is consistent with the well-accepted interpretation that slow wave gradients can determine the direction of associated propagating contractions.63 The stomach provides a precedent, in that slow wave-mediated pacemaking preferentially initiates at a specific site on the greater curvature of the corpus.64 It seems possible that there is a similar dominant pacemaker site in the human sigmoid colon, which can be activated after a meal. As we did not have sensors in the rectum in most studies, it is also possible that some of this activity originates in a more distal location (see below).
We propose that the cyclic motor patterns that occur following eating are due to an increase in extrinsic excitatory neural input, as it occurs long before the meal could have reached the colon. We postulate that extrinsic inputs, acting on enteric neural circuits, increase the amplitude of the muscle contractions revealing this myogenically coordinated retrograde motor pattern. It has previously been shown that neural input can modulate slow wave frequency65.
The physiological consequence of retrogradely propagating cyclic motor patterns
Human colonic transit studies have shown that retrograde flow is a normal component of colonic physiology. 66–68 Liquid containing bismuth infused into the rectum was observed hours later in the transverse colon37 and a magnet pill can move slowly (over several hours) in a retrograde direction from the midtransverse to ascending colon (see fig. 2 of Ref. 67). Any or all of the retrograde motor patterns described in the present study may be involved in this retrograde transport.
The prominence of retrograde cyclic propagating motor patterns in the sigmoid colon may help retard flow. In previous studies, motor complexes consisting of pressure waves with a frequency of ~3/min have been described. These rectal motor complexes or periodic rectal motor complexes17,69 have been suggested to occur in response to the arrival of stool or gas from the colon. Furthermore, manometry recordings have observed that the entire complex has been observed to move in a predominantly retrograde direction.70 It was postulated that this would act as a ‘braking mechanism’ to untimely retard the flow of colonic contents and so keep the rectum empty.70 In some of our previous work, we have shown that our cyclic motor patterns when viewed at low resolution fit the criteria for these previously described motor complexes (see Fig. 5 in Ref. 24). In our previous paper, we also proposed that absence of retrograde cyclic motor patterns in some patients may contribute to fecal incontinence.24 Furthermore, the increase in retrograde patterns in response to electrical stimulation may explain some of the symptomatic improvement shown by these patients during sacral nerve stimulation.24
This is also compatible with evidence from low resolution recordings of motor activity in the distal colon. In patients diagnosed with colitis, the amount of sigmoid phasic activity (which we now know to be largely retrograde) was inversely related to severity of diarrhea – the lower the activity, the more severe the diarrhea.71 In patients with functional diarrhea, a lack of distal colonic ‘non-propagating’ activity (sic) was associated with rapid transport of a radiopaque tracer into the recto-sigmoid after a meal.72 In both studies, the authors speculated that lack of the distal colonic motor activity contributed to the diarrhea.
Distinction of neural and myogenic pressure events by statistical analysis and the nature of ‘single’ motor patterns
Statistical analysis of the pressure events belonging to the four most common motor patterns demonstrated a stand-alone HAPS group, while the pressure events of the other three motor patterns, namely cyclic, short and long single motor patterns, could not be subdivided. As we discussed above, cyclic propagating motor patterns closely resemble the myogenic, slow wave dependent, rhythmic electrical and mechanical activity recorded from human colon in vitro.53–55 On this basis, we speculate that the pressure events that made up the ‘short single’ and ‘long single’ motor patterns share the same underlying myogenic mechanism as cyclic activity (i.e., slow waves).
The ‘long single’ propagating motor patterns described in this study resemble a distinctive form of contractile activity, which is also observed in the human colon ex vitro. Mechanical recordings of long isolated tubular specimens of human colon revealed large contractions of the circular muscle that propagated rapidly over distances up to 20 cm.73 In shorter, isolated, flatsheet preparations of human colon contractions distinctive, long-duration contractions (called ‘Slow Phasic Contractions’) were also recorded. They persisted in tetrodotoxin (i.e., myogenic in origin), and occurred at intervals of greater than 1 min.54 Importantly, these large spontaneous contractions could be prematurely triggered by activating enteric neural excitatory pathways.54 Thus, both long and short single motor patterns may reflect myogenic contractions whose incidence and site of initiation are influenced by enteric neural activity. It seems unlikely that a single myogenic contraction could travel in isolation; therefore, the propagation of both long single and short single contractions is likely to be dependent on enteric neural activity. The nature of the neural control of these two colonic motor patterns will require further investigation.
Key Messages.
Previous publications of colonic motor patterns, recorded by low-resolution manometry, distinguish high-amplitude propagating sequences (contractions), low-amplitude propagating events, an abundance of non-propagating contractions, and rarely episodes of retrograde propagating events.
Using high-resolution, fiber-optic manometry, we have re-examined this classification of motility patterns in 10 healthy adults.
Our data show that many non-propagating events are, in fact, contractions that propagate over short distances, often in the retrograde direction.
Using discriminant, logistic, and cluster analysis of the pressure event characteristics (amplitude, duration, gradient) that make up propagating motor patterns, we have shown the existence of two distinct groups, possibly indicating their neurogenic or myogenic mechanistic origins.
These data provide an in-depth basis against which patient's data can be compared.
Acknowledgments
FUNDING
Work in this grant is supported by the National Health and Medical Research Council of Australia (ID: 630502 & 1064835). Mr Wiklendt receives salary support from the Australian Research Council (ID: DP120102192) and the Clinicians Special Purpose Trust Fund of Flinders Medical Centre. Dr O'Grady receives partial salary support from National Institutes of Health (NIH) (R01 DK64775).
Footnotes
CONFLICTS OF INTEREST
None of the authors have competing interests.
AUTHOR CONTRIBUTION
PD was responsible for study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content; LW wrote the software for the analysis of manometry data, and contributed to the statistical analysis and graphical representation of data; LM was responsible for recruitment of volunteers, hospital admissions, acquisition of data in Flinders Medical Centre; VP was responsible for recruitment of volunteers, hospital admissions, acquisition of data in St. George Hospital; IG contributed to Discriminant, logistic, and cluster analysis, critical manuscript review; JA was responsible for fiber-optic catheter design and development, interpretation of manometric signals; PB and DL were responsible for Colonoscopic placement of catheters at Flinders medical Centre, and St. George Hospital, respectively, overall clinical supervision during recording made within the hospitals, and Manuscript review; GO'G, SB, and MC contributed to manuscript draft, interpretation of manometric data, and critical manuscript review.
REFERENCES
- 1.Costa M, Furness JB. The peristaltic reflex: an analysis of the nerve pathways and their pharmacology. Naunyn Schmiedebergs Arch Pharmacol. 1976;294:47–60. doi: 10.1007/BF00692784. [DOI] [PubMed] [Google Scholar]
- 2.Crema A, Frigo GM, Lecchini S. A pharmacological analysis of the peristaltic reflex in the isolated colon of the guinea-pig or cat. Br J Pharmacol. 1970;39:334–45. doi: 10.1111/j.1476-5381.1970.tb12897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Antona G, Hennig GW, Costa M, Humphreys CM, Brookes SJ. Analysis of motor patterns in the isolated guinea-pig large intestine by spatiotemporal maps. Neurogastroenterol Motil. 2001;13:483–92. doi: 10.1046/j.1365-2982.2001.00282.x. [DOI] [PubMed] [Google Scholar]
- 4.Hennig GW, Gregory S, Brookes SJ, Costa M. Non-peristaltic patterns of motor activity in the guinea-pig proximal colon. Neurogastroenterol Motil. 2010;22:e207–17. doi: 10.1111/j.1365-2982.2009.01453.x. [DOI] [PubMed] [Google Scholar]
- 5.Dinning PG, Costa M, Brookes SJ, Spencer NJ. Neurogenic and myogenic motor patterns of rabbit proximal, mid and distal colon. Am J Physiol Gastrointest Liver Physiol. 2012;303:G83–92. doi: 10.1152/ajpgi.00429.2011. [DOI] [PubMed] [Google Scholar]
- 6.Lentle RG, Janssen PW, Asvarujanon P, Chambers P, Stafford KJ, Hemar Y. High-definition spatiotemporal mapping of contractile activity in the isolated proximal colon of the rabbit. J Comp Physiol B. 2008;178:257–68. doi: 10.1007/s00360-007-0217-9. [DOI] [PubMed] [Google Scholar]
- 7.Hertz AF, Newton A. The normal movements of the colon in man. J Physiol. 1913;47:57–65. doi: 10.1113/jphysiol.1913.sp001613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hertz AF. The ileo-caecal sphincter. J Physiol. 1913;47:54–6. doi: 10.1113/jphysiol.1913.sp001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hertz AF. The pathology and treatment of chronic constipation. Proc R Soc Med. 1908;1:119–49. [PMC free article] [PubMed] [Google Scholar]
- 10.Holzknechtg G. Die normale Persistatlik des Kolon. Muench Med Wochenschr. 1909;47:2401–3. [Google Scholar]
- 11.Dinning PG, Benninga MA, Southwell BR, Scott SM. Paediatric and adult colonic manometry: a tool to help unravel the pathophysiology of constipation. World J Gastroenterol. 2010;16:5162–72. doi: 10.3748/wjg.v16.i41.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardcastle JD, Mann CV. Study of large bowel peristalsis. Gut. 1968;9:512–20. doi: 10.1136/gut.9.5.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritchie JA, Ardran GM, Truelove SC. Motor activity of the sigmoid colon of humans. A combined study by intraluminal pressure recording and cineradiography. Gastroenterology. 1962;43:642–68. [PubMed] [Google Scholar]
- 14.Torsoli A, Ramorino ML, Ammaturo MV, Capurso L, Paoluzi P, Anzini F. Mass movements and intracolonic pressures. Am J Dig Dis. 1971;16:693–6. doi: 10.1007/BF02239591. [DOI] [PubMed] [Google Scholar]
- 15.Narducci F, Bassotti G, Gaburri M, Morelli A. Twenty four hour manometric recording of colonic motor activity in healthy man. Gut. 1987;28:17–25. doi: 10.1136/gut.28.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bassotti G, Gaburri M. Manometric investigation of high-amplitude propagated contractile activity of the human colon. Am J Physiol. 1988;255:G660–4. doi: 10.1152/ajpgi.1988.255.5.G660. [DOI] [PubMed] [Google Scholar]
- 17.Rao SS, Sadeghi P, Beaty J, Kavlock R, Ackerson K. Ambulatory 24-h colonic manometry in healthy humans. Am J Physiol Gastrointest Liver Physiol. 2001;280:G629–39. doi: 10.1152/ajpgi.2001.280.4.G629. [DOI] [PubMed] [Google Scholar]
- 18.Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ, Cook IJ. Prolonged multi-point recording of colonic manometry in the unprepared human colon: providing insight into potentially relevant pressure wave parameters. Am J Gastroenterol. 2001;96:1838–48. doi: 10.1111/j.1572-0241.2001.03924.x. [DOI] [PubMed] [Google Scholar]
- 19.Bassotti G, de Roberto G, Castellani D, Sediari L, Morelli A. Normal aspects of colorectal motility and abnormalities in slow transit constipation. World J Gastroenterol. 2005;11:2691–6. doi: 10.3748/wjg.v11.i18.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arkwright JW, Underhill ID, Maunder SA, Blenman N, Szczesniak MM, Wiklendt L, Cook IJ, Lubowski DZ, et al. Design of a high-sensor count fibre optic manometry catheter for invivo colonic diagnostics. Opt Express. 2009;17:22423–31. doi: 10.1364/OE.17.022423. [DOI] [PubMed] [Google Scholar]
- 21.Dinning PG, Arkwright JW, Costa M, Wiklendt L, Hennig G, Brookes SJ, Spencer NJ. Temporal relationships between wall motion, intraluminal pressure, and flow in the isolated rabbit small intestine. Am J Physiol Gastrointest Liver Physiol. 2011;300:G577–85. doi: 10.1152/ajpgi.00532.2010. [DOI] [PubMed] [Google Scholar]
- 22.Costa M, Wiklendt L, Arkwright JW, Spencer NJ, Omari T, Brookes SJ, Dinning PG. An experimental method to identify neurogenic and myogenic active mechanical states of intestinal motility. Front Syst Neurosci. 2013;7:7. doi: 10.3389/fnsys.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dinning PG, Hunt L, Arkwright JW, Patton V, Szczesniak MM, Wiklendt L, Davidson JB, Lubowski DZ, et al. Pancolonic motor response to subsensory and suprasensory sacral nerve stimulation in patients with slow transit constipation. Br J Surg. 2012;99:1002–10. doi: 10.1002/bjs.8760. [DOI] [PubMed] [Google Scholar]
- 24.Patton V, Wiklendt L, Arkwright JW, Lubowski DZ, Dinning PG. The effect of sacral nerve stimulation on distal colonic motility in patients with faecal incontinence. Br J Surg. 2013;100:959–68. doi: 10.1002/bjs.9114. [DOI] [PubMed] [Google Scholar]
- 25.Dinning PG, Wiklendt L, Gibbins I, Patton V, Bampton PA, Lubowski DZ, Cook IJ, Arkwright JW. Lowresolution colonic manometry leads to a gross mis-interpretation of the frequency and polarity of propagating sequences: initial results from fibreoptic high-resolution manometry studies. Neurogastroenterol Motil. 2013;25:e640–9. doi: 10.1111/nmo.12170. [DOI] [PubMed] [Google Scholar]
- 26.Dinning PG, Fuentealba SE, Kennedy ML, Lubowski DZ, Cook IJ. Sacral nerve stimulation induces pan-colonic propagating pressure waves and increases defecation frequency in patients with slow-transit constipation. Colorectal Dis. 2007;9:123–32. doi: 10.1111/j.1463-1318.2006.01096.x. [DOI] [PubMed] [Google Scholar]
- 27.Wiklendt L, Mohammed SD, Scott SM, Dinning PG. Classification of normal and abnormal colonic motility based on cross-correlations of pancolonic manometry data. Neurogastroenterol Motil. 2013;25:e215–23. doi: 10.1111/nmo.12077. [DOI] [PubMed] [Google Scholar]
- 28.Everitt BS, Landau S, Leese M. Cluster Analysis. 4th edn. Arnold; London: 2001. [Google Scholar]
- 29.Norusis MJ. Inc S. SPSS Professional Statistics 6.1. Englewood Cliffs; Prentice Hall: 1994. [Google Scholar]
- 30.Hosmer DW, Lemeshow S. Applied Logistic Regression. Wiley; New Jersey: 2004. [Google Scholar]
- 31.Tabachnick BG, Fidell LS. Using Multivariate Statistics. Pearson Education; Boston, MA: 2013. [Google Scholar]
- 32.Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ, deCarle D, Cook IJ. Spatial and temporal organization of pressure patterns throughout the unprepared colon during spontaneous defecation. Am J Gastroenterol. 2000;95:1027–35. doi: 10.1111/j.1572-0241.2000.01839.x. [DOI] [PubMed] [Google Scholar]
- 33.Jouet P, Coffin B, Lemann M, Gorbatchef C, Franchisseur C, Jian R, Rambaud JC, Flourie B. Tonic and phasic motor activity in the proximal and distal colon of healthy humans. Am J Physiol Gastrointest Liver Physiol. 1998;274:G459–64. doi: 10.1152/ajpgi.1998.274.3.G459. [DOI] [PubMed] [Google Scholar]
- 34.Furukawa Y, Cook IJ, Panagopoulos V, Mcevoy RD, Sharp DJ, Simula M. Relationship between sleep patterns and human colonic motor patterns. Gastroenterology. 1994;107:1372–81. doi: 10.1016/0016-5085(94)90539-8. [DOI] [PubMed] [Google Scholar]
- 35.Hagger R, Kumar D, Benson M, Grundy A. Periodic colonic motor activity identified by 24-h pancolonic ambulatory manometry in humans. Neurogastroenterol Motil. 2002;14:271–8. doi: 10.1046/j.1365-2982.2002.00331.x. [DOI] [PubMed] [Google Scholar]
- 36.Dinning PG, Zarate N, Hunt LM, Fuentealba SE, Mohammed SD, Szczesniak MM, Lubowski DZ, Preston SL, et al. Pancolonic spatiotemporal mapping reveals regional deficiencies in, and disorganization of colonic propagating pressure waves in severe constipation. Neurogastroenterol Motil. 2010;22:e340–9. doi: 10.1111/j.1365-2982.2010.01597.x. [DOI] [PubMed] [Google Scholar]
- 37.Hertz AF. The passage of food along the human alimentary canal. Guy's Hosp Rep. 1907;61:389–427. [Google Scholar]
- 38.Costa M, Dodds KN, Wiklendt L, Spencer NJ, Brookes SJ, Dinning PG. Neurogenic and myogenic motor activity in the colon of the guinea pig, mouse, rabbit and rat. Am J Physiol Gastrointest Liver Physiol. 2013;305:G749–59. doi: 10.1152/ajpgi.00227.2013. [DOI] [PubMed] [Google Scholar]
- 39.Sarna SK, Prasad KR, Lang IM. Giant migrating contractions of the canine cecum. Am J Physiol. 1988;254:G595–601. doi: 10.1152/ajpgi.1988.254.4.G595. [DOI] [PubMed] [Google Scholar]
- 40.De Schryver AM, Samsom M, Smout AI. Effects of a meal and bisacodyl on colonic motility in healthy volunteers and patients with slow-transit constipation. Dig Dis Sci. 2003;48:1206–12. doi: 10.1023/a:1024178303076. [DOI] [PubMed] [Google Scholar]
- 41.Dinning PG, Bampton PA, Andre J, Kennedy ML, Lubowski DZ, King DW, Cook IJ. Abnormal predefecatory colonic motor patterns define constipation in obstructed defecation. Gastroenterology. 2004;127:49–56. doi: 10.1053/j.gastro.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 42.Dinning PG, Zarate N, Szczesniak MM, Mohammed SD, Preston SL, Fairclough PD, Lunniss PJ, Cook IJ, et al. Bowel preparation affects the amplitude and spatiotemporal organization of colonic propagating sequences. Neurogastroenterol Motil. 2010;22:633–e176. doi: 10.1111/j.1365-2982.2010.01480.x. [DOI] [PubMed] [Google Scholar]
- 43.Jouet P, Sabate JM, Coffin B, Lemann M, Jian R, Flourie B. Fermentation of starch stimulates propagated contractions in the human colon. Neurogastroenterol Motil. 2011;23:450–6. e176. doi: 10.1111/j.1365-2982.2010.01652.x. [DOI] [PubMed] [Google Scholar]
- 44.Kamm MA, van der Sijp JR, Lennard-Jones JE. Observations on the characteristics of stimulated defaecation in severe idiopathic constipation. Int J Colorectal Dis. 1992;7:197–201. doi: 10.1007/BF00341220. [DOI] [PubMed] [Google Scholar]
- 45.Cook IJ, Furukawa Y, Panagopoulos V, Collins PJ, Dent J. Relationships between spatial patterns of colonic pressure and individual movements of content. Am J Physiol Gastrointest Liver Physiol. 2000;278:G329–41. doi: 10.1152/ajpgi.2000.278.2.G329. [DOI] [PubMed] [Google Scholar]
- 46.Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil. 2008;20(Suppl. 1):54–63. doi: 10.1111/j.1365-2982.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 47.Rae MG, Khoyi MA, Keef KD. Modulation of cholinergic neuromuscular transmission by nitric oxide in canine colonic circular smooth muscle. Am J Physiol. 1998;275:G1324–32. doi: 10.1152/ajpgi.1998.275.6.G1324. [DOI] [PubMed] [Google Scholar]
- 48.Schang JC, Hemond M, Hebert M, Pilote M. Changes in colonic myoelectric spiking activity during stimulation by bisacodyl. Can J Physiol Pharmacol. 1986;64:39–43. doi: 10.1139/y86-005. [DOI] [PubMed] [Google Scholar]
- 49.Taylor I, Duthie HL, Smallwood R, Brown BH, Linkens D. The effect of stimulation on the myoelectrical activity of the rectosigmoid in man. Gut. 1974;15:599–607. doi: 10.1136/gut.15.8.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hara Y, Kubota M, Szurszewski JH. Electrophysiology of smooth muscle of the small intestine of some mammals. J Physiol. 1986;372:501–20. doi: 10.1113/jphysiol.1986.sp016022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daniel EE. Symposium on colonic function. Electrophysiology of the colon. Gut. 1975;16:298–306. doi: 10.1136/gut.16.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chow E, Huizinga JD. Myogenic electrical control activity in longitudinal muscle of human and dog colon. J Physiol. 1987;392:21–34. doi: 10.1113/jphysiol.1987.sp016767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rae MG, Fleming N, McGregor DB, Sanders KM, Keef KD. Control of motility patterns in the human colonic circular muscle layer by pacemaker activity. J Physiol. 1998;510(Pt 1):309–20. doi: 10.1111/j.1469-7793.1998.309bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carbone SE, Dinning PG, Costa M, Spencer NJ, Brookes SJ, Wattchow DA. Ascending excitatory neural pathways modulate slow phasic myogenic contractions in the isolated human colon. Neurogastroenterol Motil. 2013;25:670–6. doi: 10.1111/nmo.12129. [DOI] [PubMed] [Google Scholar]
- 55.Auli M, Martinez E, Gallego D, Opazo A, Espin F, Marti-Gallostra M, Jimenez M, Clave P. Effects of excitatory and inhibitory neurotransmission on motor patterns of human sigmoid colon in vitro. Br J Pharmacol. 2008;155:1043–55. doi: 10.1038/bjp.2008.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snape WJ, Matarazzo SA, Cohen S. Effect of eating and gastrointestinal hormones on human colonic myoelectrcal and motor activity. Gastroenterology. 1978;75:373–8. [PubMed] [Google Scholar]
- 57.Sullivan MA, Cohen S, Snape WJ., Jr Colonic myoelectrical activity in irritable-bowel syndrome. Effect of eating and anticholinergics. N Engl J Med. 1978;298:878–83. doi: 10.1056/NEJM197804202981604. [DOI] [PubMed] [Google Scholar]
- 58.Wright SH, Snape WJ, Jr, Battle W, Cohen S, London RL. Effect of dietary components on gastrocolonic response. Am J Physiol. 1980;238:G228–32. doi: 10.1152/ajpgi.1980.238.3.G228. [DOI] [PubMed] [Google Scholar]
- 59.Bassotti G, Betti C, Imbimbo BP, Pelli MA, Morelli A. Colonic motor response to eating: a manometric investigation in proximal and distal portions of the viscus in man. Am J Gastroenterol. 1989;84:118–22. [PubMed] [Google Scholar]
- 60.Rao SS, Kavelock R, Beaty J, Ackerson K, Stumbo P. Effects of fat and carbohydrate meals on colonic motor response. Gut. 2000;46:205–11. doi: 10.1136/gut.46.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bjornsson ES, Chey WD, Ladabaum U, Woods ML, Hooper FG, Owyang C, Hasler WL. Differential 5-HT3 mediation of human gastrocolonic response and colonic peristaltic reflex. Am J Physiol. 1998;275:G498–505. doi: 10.1152/ajpgi.1998.275.3.G498. [DOI] [PubMed] [Google Scholar]
- 62.Di Lorenzo C, Flores AF, Reddy SN, Hyman PE. Use of colonic manometry to differentiate causes of intractable constipation in children. J Pediatr. 1992;120:690–5. doi: 10.1016/s0022-3476(05)80229-x. [DOI] [PubMed] [Google Scholar]
- 63.Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Ann Rev Physiol. 2006;68:307–43. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- 64.Hinder RA, Kelly KA. Human gastric pacesetter potential. Site of origin, spread, and response to gastric transection and proximal gastric vagotomy. Am J Surg. 1977;133:29–33. doi: 10.1016/0002-9610(77)90187-8. [DOI] [PubMed] [Google Scholar]
- 65.Kim TW, Koh SD, Ordog T, Ward SM, Sanders KM. Muscarinic regulation of pacemaker frequency in murine gastric interstitial cells of Cajal. J Physiol. 2003;546:415–25. doi: 10.1113/jphysiol.2002.028977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ritchie JA, Truelove SC, Ardan GM, Tuckey MS. Propulsion and retropulsion of normal colonic contents. Am J Dig Dis. 1971;16:697–704. doi: 10.1007/BF02239592. [DOI] [PubMed] [Google Scholar]
- 67.Hiroz P, Schlageter V, Givel JC, Kucera P. Colonic movements in healthy subjects as monitored by a Magnet Tracking System. Neurogastroenterol Motil. 2009;21:838–e857. doi: 10.1111/j.1365-2982.2009.01298.x. [DOI] [PubMed] [Google Scholar]
- 68.Dinning PG, Szczesniak MM, Cook IJ. Proximal colonic propagating pressure waves sequences and their relationship with movements of content in the proximal human colon. Neurogastroenterol Motil. 2008;20:512–20. doi: 10.1111/j.1365-2982.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- 69.Kumar D, Williams NS, Waldron D, Wingate DL. Prolonged manometric recording of anorectal motor activity in ambulant human subjects: evidence of periodic activity. Gut. 1989;30:1007–11. doi: 10.1136/gut.30.7.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rao SS, Welcher K. Periodic rectal motor activity: the intrinsic colonic gatekeeper? Am J Gastroenterol. 1996;91:890–7. [PubMed] [Google Scholar]
- 71.Kern F, Jr, Almy TP, Abbot FK, Bogdonoff MD. The motility of the distal colon in nonspecific ulcerative colitis. Gastroenterology. 1951;19:492–503. [PubMed] [Google Scholar]
- 72.Bazzocchi G, Ellis J, Villanueva-Meyer J, Reddy SN, Mena I, Snape W., Jr Effect of eating on colonic motility and transit in patients with functional diarrhea. Simultaneous scintigraphic and manometric evaluations. Gastroenterology. 1991;101:1298–306. doi: 10.1016/0016-5085(91)90080-5. [DOI] [PubMed] [Google Scholar]
- 73.Spencer NJ, Kyloh M, Wattchow DA, Thomas A, Sia TC, Brookes SJ, Nicholas SJ. Characterization of motor patterns in isolated human colon: are there differences in patients with slow-transit constipation? Am J Physiol Gastrointest Liver Physiol. 2012;302:G34–43. doi: 10.1152/ajpgi.00319.2011. [DOI] [PubMed] [Google Scholar]


