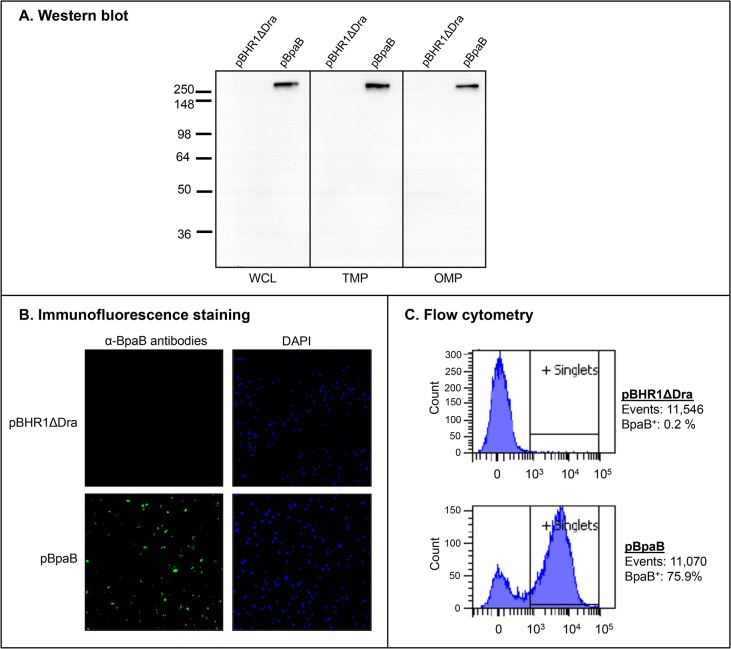
Fig 2. BpaB production by E. coli recombinant strains.
Panel A: Equivalent amounts of whole cell lysates (WCL), total membrane proteins (TMP) and sarkosyl-insoluble fractions containing OM proteins (OMP) were resolved by SDS-PAGE, transferred to PVDF membranes and analyzed by western blot with the monoclonal antibody BpaB-MAb#4. Molecular weight markers are shown to the left in kilodaltons. Panel B: Non-permeabilized E. coli strains were fixed onto glass slides and fluorescently-labeled with DAPI (blue) and with α-BpaB polyclonal antibodies (green) as described in Materials and Methods. Bacteria were visualized by microscopy using a Nikon Eclipse Ti confocal system. Representative microscopic fields are shown. Panel C: Non-permeabilized E. coli strains were incubated with polyclonal antibodies against BpaB and fluorescently-labeled with a goat α-mouse antibody conjugated with the fluorochrome Alexa Fluor 488. Labeled bacteria were analyzed using a BD LSR II flow cytometer. The x-axis represents the level of fluorescence, and the y-axis corresponds to the particles counted in arbitrary units. The number of cells analyzed, and the percentage of those producing BpaB on their surface, is shown.