Abstract
Purpose.
The cellular mechanisms linking elevated IOP with glaucomatous damage remain unresolved. Mechanical strains and short-term increases in IOP can trigger ATP release from retinal neurons and astrocytes, but the response to chronic IOP elevation is unknown. As excess extracellular ATP can increase inflammation and damage neurons, we asked if sustained IOP elevation was associated with a sustained increase in extracellular ATP in the posterior eye.
Methods.
No ideal animal model of chronic glaucoma exists, so three different models were used. Tg-MyocY437H mice were examined at 40 weeks, while IOP was elevated in rats following injection of hypertonic saline into episcleral veins and in cynomolgus monkeys by laser photocoagulation of the trabecular meshwork. The ATP levels were measured using the luciferin-luciferase assay while levels of NTPDase1 were assessed using qPCR, immunoblots, and immunohistochemistry.
Results.
The ATP levels were elevated in the vitreal humor of rats, mice, and primates after a sustained period of IOP elevation. The ecto-ATPase NTPDase1 was elevated in optic nerve head astrocytes exposed to extracellular ATP for an extended period. NTPDase1 was also elevated in the retinal tissue of rats, mice, and primates, and in the optic nerve of rats, with chronic elevation in IOP.
Conclusions.
A sustained elevation in extracellular ATP, and upregulation of NTPDase1, occurs in the posterior eye of rat, mouse, and primate models of chronic glaucoma. This suggests the elevation in extracellular ATP may be sustained in chronic glaucoma, and implies a role for altered purinergic signaling in the disease.
Keywords: ATP release, mechanosensitive, retina
Increased extracellular ATP levels and changes to ectoATPases are identified in mouse, rat and primate models of chronic glaucoma, implicating altered purinergic signaling in the response to sustained, as well as acute, elevations of IOP.
Glaucoma is one of the leading causes of blindness in humans and is characterized by damage to retinal ganglion cells (RGCs) and the optic nerve.1,2 While abnormally high IOP is a widely recognized risk factor,3,4 the molecular pathways linking elevated IOP and RGC loss are complex. Although several inroads toward understanding the mechanisms have recently been made,5–13 much remains to be learned, particularly regarding the responses in chronic injury.
Extracellular ATP is a likely candidate to link the elevated IOP in glaucoma to perturbed signaling in the retina and optic nerve. Throughout the body, released ATP conveys information about mechanical strain and accompanies shear stress, swelling, and stretch.14–18 This mechanosensitive release of ATP can initiate a series of physiological and pathological responses including cell death, volume regulation, pain, inflammatory responses, and neuroprotection by activating ionotropic P2X and metabotropic P2Y receptors.19
Evidence is accumulating for an increased release of ATP following IOP elevation in glaucoma. For instance, humans with primary acute angle closure glaucoma (PAACG) displayed a 9-fold rise in the ATP levels in their anterior chamber.20 Bovine eyes released ATP into the vitreal chamber in levels proportional to the magnitude of applied pressure,21 while the damage caused to rat RGCs by acute rises in IOP was related to levels of extracellular ATP.22 Both RGCs and astrocytes release ATP upon mechanical strain, with pannexin hemichannels a likely pathway for release in both cell types.23–25
While these reports indicate that acute increases in IOP and/or mechanical strain lead to release of ATP in the retina, most patients with glaucoma suffer from an elevation in IOP for extended periods. Evidence for a sustained release of ATP associated with a prolonged elevation of IOP would thus have relevance for the most common forms of the disease. We have previously demonstrated that humans with primary chronic angle closure glaucoma (PCACG) have a 14-fold increase in ATP levels in the anterior chamber.26 While this finding supports the theory that a chronic elevation in IOP leads to a sustained elevation in extracellular ATP release, it was not possible to confirm this increase in the human retina as sampling was only justified from the anterior segment in these patients and not in the vitreal chamber. As the pathological changes to the retina and optic nerve head are most directly associated with vision loss, it is important to determine whether elevated extracellular levels of ATP also occur in the posterior eye during a chronic rise in IOP. Given that excessive stimulation of the P2X7 receptor for ATP can both kill RGCs and activate the inflammasome,27,28 the relationship between elevated IOP and extracellular ATP in chronic glaucoma has particular importance.
The present study asked whether extracellular ATP was elevated in animal models of chronic glaucoma. As all current animal models of chronic glaucoma are imperfect, we used three different models to ensure the broadest relevance of the findings to the human condition. Specifically, chronic elevation of IOP was monitored in the Tg-MyocY437H mouse, in rat eyes following injection of hypertonic saline into episcleral veins, and in primates after laser photocoagulation of the trabecular meshwork. As ATP measurement from the small extracellular space in the retina is problematic, ATP levels in the vitreous were measured. Levels of the enzyme NTPDase1 were also compared in retinal tissue from rats, mice, and primates with sustained elevation in IOP and control IOP levels as sustained exposure to extracellular ATP increased expression of the ectoATPase NTPDase1 in optic nerve head astrocytes in vitro.
Methods
Animal Models of Chronic Glaucoma
All experiments were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research using protocols approved by the Animal Care and Use Committee of the University of Pennsylvania (Philadelphia, PA, USA) or, in the case of the primates, the University of Wisconsin (Madison, WI, USA).
Rat Episcleral Vein Scarring Model of Chronic IOP Elevation.
Unilateral elevation of IOP was produced in 12 adult male Brown Norway rats based upon protocols previously described.29 In brief, anesthesia was induced by intraperitoneal injection of a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg). Hypertonic saline (1.75–1.9 M) was injected into the episcleral vein of one eye through a glass microneedle while the other eye served as a control. A syringe pump (WPI, Sarasota, FL, USA) was used to standardize the injection pressure, volume, and duration. The procedure was successful approximately half the time when defined as resulting in a sustained elevation in IOP; results presented are only from successful interventions. The IOP was monitored with a TonoLab tonometer (Colonial Medical Supply, Franconia, NH, USA) three to four times weekly from conscious rats usually with 0.5% proparacaine hydrochloride eye drops. After 14 days of elevated IOP, animals were euthanized and eyes fast frozen. The mean IOP for the rats is calculated from the average for each rat over days 4 to 14.
Tg-MYOCY437H Transgenic Mouse Model of Chronic Glaucoma.
Tg-MyocY437H transgenic mice were generated and initially characterized by Sheffield and colleagues.30 A colony was established at the University of Pennsylvania. Tg-MyocY437H mice were bred with C56BL/6N mice and the offspring were genotyped to identify those with the human transgene. All mice had normal expression of the mouse myocilin gene; RT-PCR confirmed that mouse myocilin was present in the trabecular meshwork and iris and but not in the retina (not shown). Intraocular pressure was measured from litter mate C56BL/6N and Tg-MyocY437H mice monthly. Mice were conscious but gently restrained and IOP was measured using a TonoLab tonometer under topical anesthesia with 0.5% proparacaine hydrochloride eye drops. Each IOP value is the mean of six measurements from the left and six from the right eye, performed between 2 PM and 5 PM; daytime readings likely underestimated IOP differences30 but the small variation made the difference reliable.
Primate Laser Model of Sustained IOP Elevation.
The argon laser photocoagulation model of IOP elevation was used as described.31,32 Fourteen cynomolgus monkeys (Macaca fascularis) of either sex were treated in accordance with the ARVO Statement for Use of Animals in Ophthalmic and Vision Research, using protocols approved by the University of Wisconsin-Madison Research Animal Resources Center. Intraocular pressure was elevated unilaterally by argon laser photocoagulation of the trabecular meshwork. Laser spots were applied using a standard clinical argon laser at 1.0 to 1.5 W power. Laser pulse duration and spot size were fixed at 0.5 second and 50 μm, respectively. In some trials, animals received repeated treatment at approximately 3- to 4-week intervals, until a sustained elevation in IOP was achieved. IOP was determined using Goldmann applanation tonometer readings in intramuscular ketamine-sedated monkeys (∼10 mg/kg body weight).32 Intraocular pressure was reduced by topical application of daily timolol (Timoptic-XE; 30 μL, 0.5%, prostaglandin F2α isopropyl ester, 2 μg in 5 μL titrated as needed to maintain IOP) if pressure rose outside of the target range of 30 to 45 mm Hg. The cup-disc ratio (C/D) measure of optic nerve damage was assessed clinically in the monkeys by means of stereoscopic slit-lamp biomicroscopy and fundus photography through dilated pupils. A single unmasked examiner (PLK) estimated the C/D ratios in experimental glaucoma (Exp) and control eyes of all animals shortly before lasering and again shortly before enucleation. The mean IOP is defined as the average of all IOP readings after the initial laser treatment; as some monkeys required several treatments, IOP was often higher closer to sacrifice. Vitreal ATP measurements were obtained from a set of eight primates and the protein measurements were obtained from an additional six. The IOP was elevated in the primate eyes for 16.0 ± 2.4 weeks before the vitreal tap and subsequent sacrifice, and 19.0 ± 5.1 weeks before tissue was used for immunoblots. In two of eight monkeys used for ATP measurements, memantine (2–8 mg/kg) was given in fruit, although this had no significant effect on either ATP not IOP in these two samples.
Retinal Ganglion Cell Counts
Rat RGCs were back-labeled by the injection of the FluoroGold derivative aminostilbamidine (Invitrogen Corp., Carlsbad, CA, USA) into the superior colliculus as previously reported.33 After allowing 2 to 3 days for retrograde transport of the dye down the optic nerve to the RGC soma, eyes were enucleated, and retinal whole-mounts were examined using a fluorescence microscope (Nikon Eclipse E600, Nikon, Inc., Melville, NY, USA) after fixation with 4% paraformaldehyde. For RGC counts in mice, dissected retinas were incubated with 0.1% Triton X-100 in SuperBlock blocking buffer (ThermoFisher Scientific, Waltham, MA, USA) for 10 minutes followed by blocking with 5% goat serum in SuperBlock buffer for 1 hour. Retinas were then incubated with goat anti mouse Brn-3b antibody (1:1000; Santa Cruz Biotech, Inc., Dallas, TX, USA) overnight at 4°C, followed by incubation with rabbit anti-goat 568 secondary antibody (1:200; Invitrogen Corp.). For rodent counts, whole-mount preparations were divided into superior, nasal, inferior, and temporal quadrants and six areas per retinal quadrant were examined with a ×40 objective. The regions were located circumferentially two-sixths (central), one-half (middle), and five-sixths (peripheral) from the optic nerve rim. Areas were digitally photographed using Image Pro Plus software (Media Cybernetics, Inc., Silver Spring, MD, USA) and the RGCs were counted in each region in a masked fashion.
Vitreous Collection and ATP Measurement
In the rat, vitreous samples were collected 14 days after successful surgery. Samples were dissected in a Petri dish over dry ice to keep the cut edges frozen and prevent the release of ATP from surrounding tissue, this small step proved critical. Analogous approaches were used to obtain samples from the mouse eye although only 2.5 μL of the vitreous sample was obtained. In primates, vitreous sampling was performed as described.32 In brief, a 23-G needle attached to a tuberculin syringe was inserted through the pars plana 12 to 14 mm toward the papillomacular nerve fiber bundle under direct visualization with an operating microscope under anesthesia. Between 100 and 200 μL of vitreous was aspirated from each eye, and the vitreous cavity volume was restored with hyaluronate sodium viscoelastic material (Healon; Pharmacia Corp., Abbott Laboratories, Inc., Abbott Park, IL, USA). In all cases, vitreous samples were quickly frozen in liquid nitrogen and stored at −80°C for future assay. The ATP levels were determined with a modified version of the chemiluminescent luciferin-luciferase reaction previously described.34 In particular, 2.5 to 5 μL of the vitreal sample was added to approximately 90 μL luciferin-luciferase working solution and luminescence determined with a plate-reading luminometer (Luminoskan Ascent; Labsystems, Franklin, MA, USA). Luminescence values were calibrated to ATP concentration in mouse and monkey but not rat samples.
Immunoblots
Eyes were sectioned at the ora serrata, the vitreous was removed and the retina peeled from the RPE before being quick frozen. Retinas were washed twice with cold Dulbecco's phosphate-buffered saline and lysed in RIPA buffer containing 50 mM Tris-HCl, 150 mM NaCl, protease inhibitor cocktail (Complete; Roche Diagnostics Corp., Indianapolis, IN, USA), 1% Triton X-100, 0.1% SDS, and 10% glycerol. The samples were sonicated and cleared by centrifugation (10,000g) for 30 minutes at 4°C. Protein concentrations were determined using BCA Protein Assay. Retinal protein (20 μg) was separated using nonreduced conditions, and processed using standard Western blot protocols.35 For the myelinated optic nerve tissue of the rat, the outer pial layers were removed from 3 mm of the post scleral optic nerve and lysed in RIPA buffer; 2 μg protein was separated and processed as above. A primary rabbit polyclonal antibody against rat NTPDase1 (rN1-I4, 1:1000) was obtained from Jean Sévigny (in the public domain, http://ectonucleotidases-ab.com).36 Analogous approaches were used for mouse tissue except that 15 μg protein was used for each lane and a rabbit polyclonal antibody to mouse NTPDase1 was used (C9F, from JS; 1:1000).37 Primate NTPDase1 was identified with a mouse monoclonal antibody against human NTPDase1 (BU61, 1 μg/ml; Ancell Corp., Bayport, MN, USA). The primary antibodies were resolved by incubating blots with an IgG of the appropriate species conjugated to horseradish peroxidase (1:5000 dilution; Amersham Biosciences Corp., Arlington Heights, IL, USA) at room temperature for 1 hour and developing by chemiluminescence detection (ECL detection system; Amersham Biosciences Corp.). Bands were quantified using Image Pro Plus software (primate), a LAS-3000 Image Reader and analyzed with V4.3 software (rat; Fujifilm, Stamford, CT, USA) or an ImageQuant LAS 400 image reader (mouse, GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) and analyzed with associated software. Immunoblots for rat, mouse, and primate tissue were repeated 2 to 4 times. It is worth stressing that all antibodies against NTPDase1 used in this study (rN1-I4 for rat, C9F for mouse, and BU61 for monkey) require use of nondenatured protein. As NTPDase1 is heavily glycosylated the bands detected ran approximately 70 to 80 kDa as described.38–41 Positive controls were run for C9F with lung, for rN1-I4 in spleen and liver, and BU61 in umbilical vein endothelial cells; bands from the retina of each species ran at the same size as those in the positive control.
Quantitative PCR
Mouse retinas were rapidly homogenized in 1 mL Trizol reagent and the total RNA was purified using the RNAeasy mini kit (Qiagen, Inc., Gaithersburg, MD, USA). Oligo-dT primed first-strand cDNA was synthesized using 1 μg of total RNA per reaction (Invitrogen Corp.). Quantitative PCR (qPCR) was carried out using a Power SYBR Green master mix and the quantitative assessment of gene levels was performed using a 7300 Real-Time PCR System (Invitrogen Corp.). Primers for mouse NTPDase1 (154 bp): F: CATCCAAGCATCACCAGACT R: ATGATCTTGGCACCCTGGAA; mouse GAPDH (169bp): F: TCACCACCATGGAGAAGGC R: GCTAAGCAGTTGGTGGTGCA. To ask if prolonged elevation of extracellular ATP upregulates NTPDase1 in primary rat optic nerve astrocytes, cells were cultured as described.25 Upon confluence, the slowly hydrolysable ATP analogue ATPγS was added for 24 hours at the concentration indicated, after which RNA was extracted and used for qPCR using techniques described above. Primes for rat NTPDase142 (224bp) F: AGGAGCCTGAAGAGCTACCC R: GTCTGATTTAGGGGCACGAA; rat GAPDH (169 bp) F: TCACCACCATGGAGAAGGC; R: GCTAAGCAGTTGGTGGTGCA.
Immunohistochemistry in Primate Retina
Eyes were sectioned at 6 μm, fixed with 4% paraformaldehyde and blocked with SuperBlock Blocking Buffer for 1 hour at room temperature. Sections were incubated overnight at 4°C with the BU61 antibody (1:200), followed by incubation with an anti-mouse Cy3 secondary antibody for 60 minutes at room temperature and brief incubation with nuclear stain 4′,6-diamidino-2-phenylindole (DAPI; both ThermoFisher Scientific). Images of all slides were captured digitally with a Nikon Eclipse E600, exciting Cy3 at 562 nm and DAPI at 360 nm. No staining was observed in the absence of primary antibody.
Data Analysis
Data are reported as mean ± SEM. Statistical analysis used a paired Student's t-test was used to compare results from contralateral rat and primate eyes, or a one-way ANOVA with appropriate post hoc test as appropriate. Analysis was performed on ranks where data were not normally distributed. Analysis was performed using SigmaStat (Systat Software, Inc., San Jose, CA, USA). Results with P less than 0.05 were considered significant. Correlations were determined using a first order linear regression, with P values given determined by SigmaStat analysis.
Results
Elevated Extracellular ATP and NTPDase1 in Rats With Experimental Glaucoma
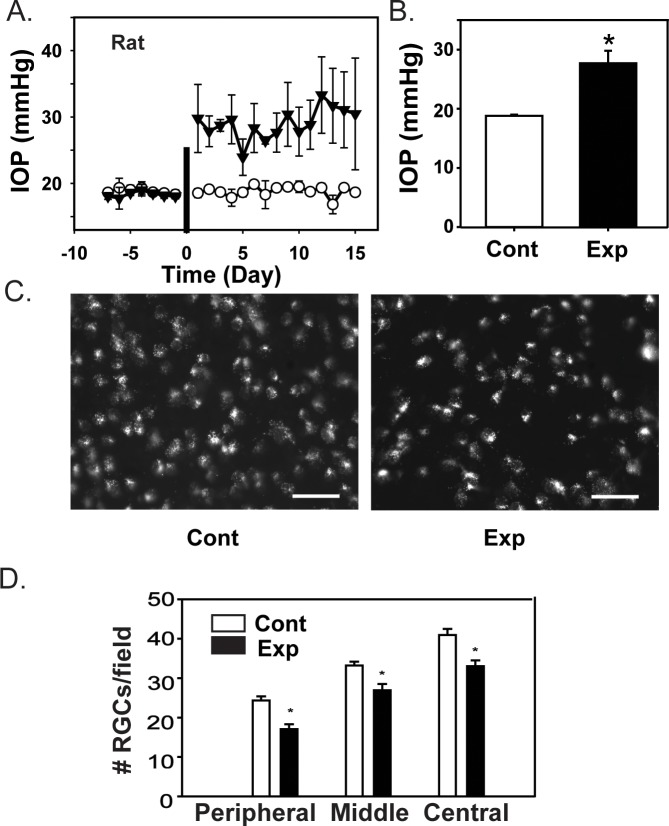
Initial experiments asked whether rats with sustained elevation of IOP had elevated levels of ATP in the posterior eye. A moderate elevation of IOP was detected following the successful injection of hypertonic saline into the rat episcleral vein. The mean IOP levels for the period before and after saline injection are shown in Figure 1A. The IOP in the experimental eyes was consistently greater than that of the control eyes, although there was variation in the magnitude of this difference from day to day. Overall, the mean IOP after injection of hypertonic saline was 27.7 ± 2.1 mm Hg for the experimental eyes, while IOP in the contralateral control eyes over the same period was 18.8 ± 0.2 mm Hg (Fig. 1B, P = 0.004).
Figure 1.
Intraocular pressure elevation in rat eyes with experimental glaucoma. Increased IOP following injection of hypertonic saline induces RGC loss in rats. (A) The temporal changes in IOP measurements from all rats, indicating the IOP levels in the control (white circles) and contralateral experimental eyes (black circles) before and after injection of hypertonic saline. The injection point is indicated with a vertical bar on day 0. Each symbol is the mean ± SEM from three to nine measurements (IOP was not measured daily from each rat). (B) Mean values of IOP. There was a significant difference in the mean IOP between experimental (Exp) and control (Cont) eyes (n = 9 pairs, each a mean of measurements taken on 3–11 days, P = 0.004, paired t-test). (C) Representative images from a pair of eyes with control (Cont) and experimentally elevated levels of IOP (Exp), showing RGCs loaded with aminostilbamidine for cell counting. Images were obtained in the peripheral area of the nasal quadrant, one visual field from the retinal edge. A reduced density of fluorescent ganglion cells was apparent in the experimental eye, scale bars: 50 μm. (D) Quantification of RGCs after 14 days of elevated IOP. A significant reduction in cell number was present in all three retinal regions (n = 3, P = 0.002, 0.010, and <0.001 respectively for peripheral, middle, and central regions).
To determine if elevated IOP damaged ganglion cells in the rat retina, we quantified RGC numbers in all four quadrants of retina using retrograde labeling with aminostilbamidine.27 Retinal ganglion cells were identified by the characteristic fluorescence pattern consistent with the transport of the dye. Cell density was significantly reduced in experimental eyes as compared with controls (Fig. 1C). Overall, the mean cell counts were reduced by 29.8% in the peripheral region of the retina (P = 0.002), by 18.9% in the middle region (P = 0.010), and by 19.2% in the central region (P < 0.001) as compared with healthy control eyes (Fig. 1D). This implied that the rise in IOP induced by injection of hypertonic saline reduced the viability of RGCs, or their ability to transport aminostilbamidine.
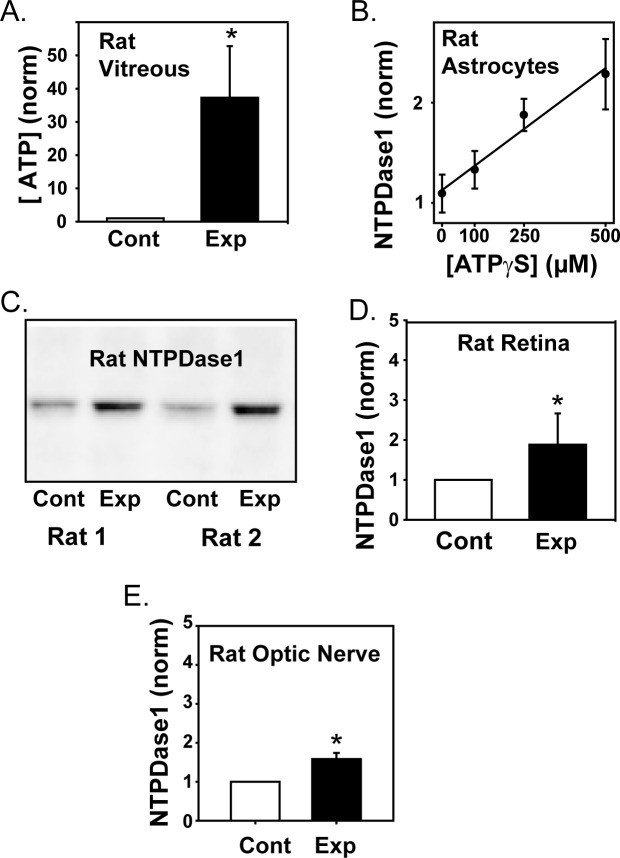
Levels of extracellular ATP were determined in the posterior rat eye. Two precautions were taken to avoid artifactual contamination by ATP from intracellular sources. First, ATP levels were measured from the acellular posterior vitreous. While the absolute magnitude of ATP diffused into the vitreal chamber was low, the relative rise was substantial, and this avoided any chance of contamination from ruptured or prodded cells within the retina while obtaining a sample. Second, vitreal samples were obtained from frozen eyes dissected over dry ice. Given the large intracellular ATP concentration, the use of frozen eyes prevented seepage of ATP from the cut cells of the peripheral eye. The ATP levels were higher in extracellular vitreal solution from eyes with sustained elevated IOP as compared with contralateral control eyes (Fig. 2A, P = 0.039). There was a significant correlation between the rise in ATP levels, as normalized to the control eye for each pair, and the IOP levels, defined as the mean value in the 10 to 14 days preceding euthanization (P = 0.005).
Figure 2.
The ATP levels in retina of rats with experimental glaucoma. (A) The concentrations of ATP in the vitreous of rats with experimentally increased IOP (Exp) was greater than in contralateral control eyes (Cont; n = 8, P = 0.039). Levels were normalized to the luminescence output from the luciferase assay in the control eye of each pair. (B) Exposure of rat optic nerve head astrocytes to ATPγS in the bath led to a dose dependent rise in mRNA for NTPDase1, with data fit by a first order regression (P = 0.015). (C) Representative immunoblots of two eye pairs with anti-NTPDase1 antibody demonstrating the increase in protein levels in retinas from experimental eyes with increased IOP as compared with contralateral controls. Blots were detected at the expected size of 72 to 75 kDa for the glycosylated protein in rat. (D) Mean elevation in NTPDase1 protein levels in retinas from eyes with elevated IOP. Blot intensities were normalized to the control of each pair (P = 0.016, n = 8). (E) NTPDase1 expression as determined from densitometry analysis of immunoblots increased in optic nerves from eyes with elevated IOP (Exp) as compared with contralateral control nerves (Cont; P = 0.002, n = 6).
While direct measurement of vitreal ATP suggested an increased ATP release accompanied IOP elevation, confirmation was sought. The extracellular enzyme NTPDase1 was previously shown to be upregulated in retinal pigmented epithelial cells on a molecular and protein level after sustained exposure to elevated levels of extracellular ATP.35 To determine whether NTPDase1 also reflected the extracellular ATP concentration in cells relevant for glaucoma, rat cultured optic nerve head astrocytes were exposed to the slowly hydrolysable ATP analogue ATPγS in the bath at concentrations of 0, 100, 250, and 500 μM for 24 hours, after which the expression of NTPDase1 mRNA was determined with qPCR. As shown in Figure 2B, the rise in NTPDase1 expression closely reflected the level of extracellular ATPγS in optic nerve head astrocytes (P = 0.015).
NTPDase1 levels in the rat retina were determined. Immunoblots from retina of the control and experimental eyes indicated that levels of NTPDase1 protein were higher in tissues from eyes with elevated IOP (Fig. 2C). Quantification indicated that the relative amount of NTPDase1 in the experimental eye was twice that in the contralateral control eye (Fig. 2D, P = 0.003). The rise in NTPDase1 was correlated with the rise in IOP (P = 0.003, n = 6) and vitreal ATP levels (P < 0.001, n = 6).
The retinal tissue used in these studies includes the optic nerve head, where much of the disruption in glaucoma is thought to occur. However, damage can also extend past the optic nerve head into the myelinated portion of the nerve. Immunoblots were thus performed to evaluate the expression of the NTPDase1 in the retrobulbar optic nerve of rats with increased IOP, using the 3 mm of nerve extending distally from the sclera. NTPDase1 protein expression was increased in the optic nerve of eyes with elevated IOP as compared with contralateral controls (Fig. 2E, P = 0.002). The relative increase in NTPDase1 levels in the optic nerve was also correlated with the magnitude of IOP elevation (P = 0.035).
Elevation of Vitreal ATP and Retinal NTPDase1 in the Tg-MyocY437H Mouse Model
To determine whether the relationship between elevated IOP and increased extracellular ATP applied to other models of chronic glaucoma, the study was extended to the transgenic Tg-MyocY437H mice.30 The IOP increased steadily from 10 to 40 weeks in mice with the MyocY437H transgene as compared with wild-type controls (Fig. 3A). At 40 weeks, the IOP in the Tg-MyocY437H mice showed a modest but significant rise in IOP as compared with controls, at 15.64 ± 0.26 vs. 12.14 ± 0.15 (P < 0.001; n = 16, 12, respectively). These IOP levels were measured during the day, and the rise in IOP is smaller than that observed with nocturnal measurements,30 as expected. To determine if this rise in IOP was associated with RGC loss, retinal whole-mounts were stained with an antibody to RGC marker Brn-3b (Fig. 3B) and the surviving cells counted in the central, middle, and peripheral regions as performed for rat. There was a 25.2% drop in RGC number in the peripheral region (P < 0.001) and a 12.9% drop in RGCs the middle region (P = 0.020) of 40-week-old Tg-MyocY437H mice as compared with controls (Fig. 3C).
Figure 3.
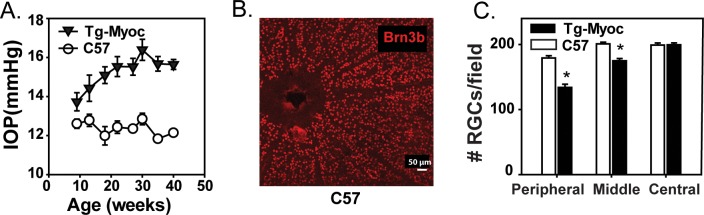
Intraocular pressure and RGCs in retina of Tg-MyocY437H mice. (A) Intraocular pressure levels from Tg-MyocY437H mice (black triangles) as compared with sibling wild-types (white circles) measured every 4 weeks (mean ± SEM, P = 0.008, paired t-test for each time point, n = 12–16 eyes). (B) Typical whole mount of control retina stained with Brn-3b antibody showing distinct RGC soma used for quantification, scale bar: 50 μm. (C) Mean RGCs per field in peripheral (P < 0.001), middle (P = 0.020), and central (NS) retinal regions in 40-week-old wild-type (white bars) versus Tg-MyocY437H mice (black bars); (n = 3 mice, 8 counts per region).
The ATP concentration in the vitreous of Tg-MyocY437H mice was significantly elevated as compared with age-matched wild-type controls at 7 to 16 months (Fig. 4A). The absolute levels rose from 9.6 ± 1.3 nM in the wild-type eyes to 26.0 ± 7.8 nM in the eyes from the transgenic mice (P = 0.033); the low absolute levels likely reflect the diffusion distance from the cellular tissue to where the sample was obtained in the vitreal cavity (see Methods). The ATP concentration was significantly correlated to the IOP levels (P = 0.013).
Figure 4.
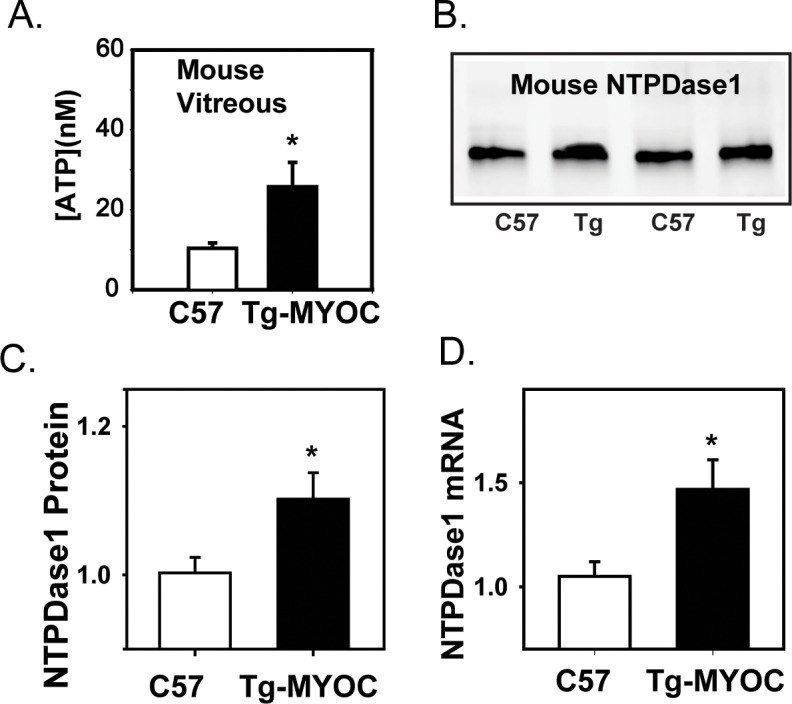
Increase in ATP and NTPDase1 in Tg-MyocY437H mice. (A) The concentration of ATP in the vitreous of Tg-MyocY437H mice at 7 to 16 months was significantly higher than in age-matched sibling controls (n = 6, P = 0.034). (B) Representative immunoblots from retinas of two 40-week-old Tg-MyocY437H mice and two age-matched controls showing increased NTPDase1 bands in the Tg-MyocY437H mice. Bands were at the predicted 72 to 75 kDa of the glycosylated protein in mice. (C) Mean NTPDase1 protein levels quantified from immunoblots indicates an increase in Tg-MyocY437H mice (P = 0.031, n = 11–12). Message levels normalized to mean control levels. (D) Quantitative PCR results demonstrate an increase in mRNA message for NTPDase1 in retina for 40-week-old Tg-MyocY437H mice as compared with control (P = 0.029, n = 6–7). Message levels normalized to mean control levels.
Immunoblots confirmed a small but significant rise in NTPDase1 from retinal tissue at the protein level (Fig. 4B, C; P = 0.031). The relative rise in NTPDase1 protein also correlated with the IOP measured at 40 weeks (P = 0.012). Given that this rise was modest, levels of NTPDase1 mRNA were determined from mouse retinal tissue using qPCR to support the increase in NTPDase1 protein. Message for NTPDase1 was significantly increased by 41.1% in the retina of 40-week-old Tg-MyocY437H mice as compared with age-matched wild-type controls (P = 0.029; Fig. 4D).
Elevation of Vitreal ATP and Retinal NTPDase1 in Monkeys With Experimental Glaucoma
While measures in rat and mouse support the link between increased extracellular ATP and sustained elevation of IOP, primate eyes were examined as they have considerably greater relevance to the glaucomatous condition in humans. Intraocular pressure was elevated in one eye of 14 cynomolgus monkeys using argon photo coagulation. An example of the time-dependent changes in IOP is shown in Figure 5A. The mean IOP in the treated eyes was 32.6 ± 2.6 mm Hg as compared with 16.8 ± 0.5 mm Hg in the untreated contralateral eyes (P < 0.001; Fig. 5B). The mean C/D ratio determined before euthanization was 0.21 ± 0.05 in untreated eyes and 0.53 ± 0.14 in the lasered eyes (P = 0.043; Fig. 5C), and was correlated with the change in IOP in each animal (P = 0.026). The quantity of RGC marker Brn-3b was decreased by 53% in retinal immunoblots from experimental eyes compared with the untreated contralateral eyes (P = 0.002; Fig. 5D). Levels of Brn-3b were significantly correlated with IOP (P = 0.013).
Figure 5.
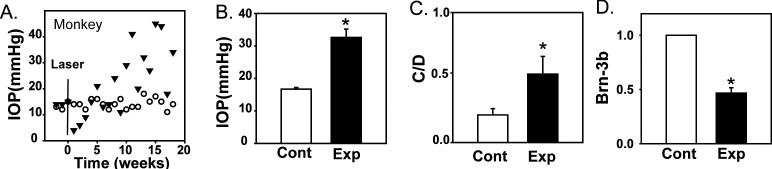
Intraocular pressure elevation in primate eyes with experimental glaucoma. (A) Intraocular pressure measurements from the control eye (white circles) and contralateral eye of a monkey that underwent photocoagulation of the trabecular meshwork (black triangles) before and after treatment on week 0 (black line). Timolol was given on weeks 17 and 18 to the treated eye to reduce the pressure. (B) The mean IOP from the experimental treated eye (Exp) of monkeys used in this study was significantly higher than in the untreated contralateral control eye (Cont; P = 0.006, n = 6). (C) Cup-disc ratios of experimental and contralateral control eyes (P = 0.043, n = 6). (D) The intensity of RGC marker Brn-3b in immunoblots from experimental eyes was reduced as compared with the contralateral control eye (P = 0.002, n = 6).
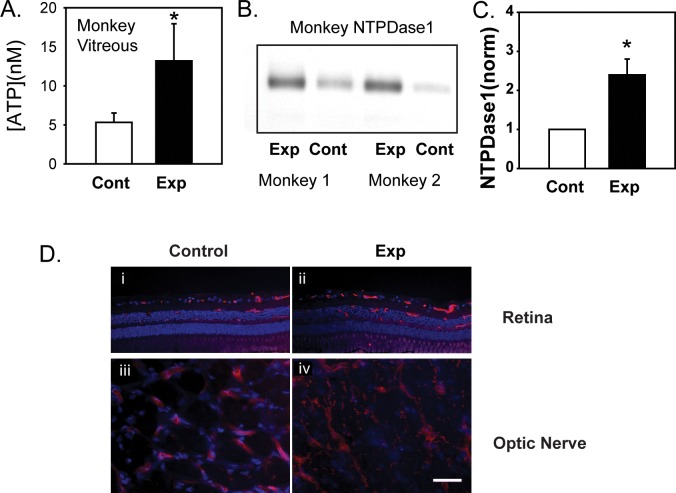
The ATP levels were measured from vitreal samples to determine whether primate retinas with chronic elevation in IOP had a sustained increase in extracellular ATP. The ATP levels were significantly increased in eyes with elevated IOP, rising from 5.3 ± 1.2 nM in control to 13.2 ± 4.8 in the treated eye (Fig. 6A, n = 8; P = 0.039). In another set of eyes, whole retinal protein lysates were immunoblotted for NTPDase1 (Fig. 6B). Bands were more intense in material from retinas of monkey eye with elevated IOP. Of the six pairs examined, levels increased by a mean of 2.4 ± 0.4–fo1d (Fig. 6C, P = 0.018). The relative rise in NTPDase1 in the experimental eye was correlated with the rise in IOP (P = 0.027). Immunohistochemical staining for NTPDase1 was performed to localize the rise in NTPDase1 (Fig. 6D). In eyes with elevated IOP, there was an increase in diffuse irregular staining throughout the inner retina (Fig. 6Dii vs. i) and optic nerve (Fig. 6Div vs. iii), although the cell type was not identified.
Figure 6.
NTPDase1 levels in primates with experimental glaucoma. (A) The ATP levels are increased in the vitreous of primates with increased IOP compared with the contralateral controls (n = 8, P = 0.039). (B) Representative immunoblots for NTPDase1 of experimental (Exp) and contralateral untreated control retinas (Cont) from two monkeys indicating increase in NTPDase1 protein levels in retinas from eyes with experimental glaucoma. Bands were at the predicted size of the glycosylated proteins in primates (75–80 kDa). (C) Quantification of NTPDase1 protein using densitometry analysis of immunoblots shows retinas from eyes with increased IOP (Exp) had significantly increased levels of NTPDase1 as compared with contralateral controls (Cont; P = 0.018, n = 6 in triplicate). (D) Immunohistochemical staining for NTPDase1 in retina (i, ii) and optic nerve (iii, iv) from eyes with elevated IOP (Exp) and the contralateral control (Cont), scale bar: 10 μm.
Discussion
This study used rat, mouse, and primate models of chronic glaucoma to ask if sustained elevation of IOP was associated with a sustained elevation in extracellular ATP. In all three models, data suggest that extracellular ATP remains elevated in the posterior eye throughout the period of increased IOP.
The rat hypertonic saline model, the Tg-MyocY437H mouse model and the monkey laser photocoagulation model are three different approaches used to create a sustained elevation in IOP. While each model has its advantages and disadvantages, the observation that extracellular ATP was elevated in the affected eyes of all three models provides strong support for the link between sustained increase in IOP and sustained rise in extracellular ATP. When combined with previous data showing elevated ATP levels in the aqueous humor of humans with primary chronic glaucoma,26 this suggests there may be a sustained elevation of extracellular ATP in the posterior eye of patients with a chronic elevation of IOP.
Short-term increases in ocular pressure have previously been shown to elevate ATP levels in the vitreal chambers of rat22 and bovine retina,21 and in the aqueous humor of humans.43 Mechanical strain induces ATP release through pannexin channels located on RGCs23 and optic nerve head astrocytes.25 While the acute response is reasonably well established, it was important to determine whether there was a sustained elevation in extracellular ATP as many forms of glaucoma progresses over a time course of years.44–46 Given that a loss of RGCs and an elevation of IOP were demonstrated in all three models used here, the increases in extracellular ATP likely result from a combination of mechanosensitive release and cell rupture.
Several technical details are worth noting. The first is the elevation of NTPDase1 after prolonged exposure to extracellular ATP. This was previously demonstrated in RPE cells, with exposure to the slowly-hydrolysable agonist ATPγS increasing NTPDase1 on an mRNA and protein level over 12 to 48 hours.35 The present study suggests that expression of NTPDase1 in rat optic nerve head astrocytes is elevated upon exposure to ATPγS for 24 hours. NTPDase1 is an ectoenzyme, which dephosphorylates both ATP and ADP,47 and thus upregulation in response to a prolonged exposure to substrate represents simple negative feedback. In the rat model of chronic IOP elevation (the only model for which comparisons were possible), extracellular ATP levels were tightly correlated with NTPDase1 levels (P < 0.001), suggesting this linkage occurs in vivo as well as in vitro. This linkage is important because the direct measurement of extracellular ATP in tissues with a confined extracellular space is complicated by the high levels of intracellular ATP that can contaminate samples upon cell rupture and by the mechanosensitive release of ATP that accompanies the prodding advance of a probe. While it is unclear whether elevated levels of NTPDase1 can serve as an actual marker for sustained elevation in extracellular ATP concentration in this study, the rise of NTPDase1 in rat, mouse, and primate models indicates a generalized perturbation in purinergic signaling occurs with chronic glaucoma.
The second issue involves the localization of this increased extracellular ATP. The absolute concentrations of ATP measured in mouse and monkey vitreous are low, but as the nearest cell was some distance from the sampling point, these concentrations are likely diluted several fold from levels in the retina. While the cells responsible for this release in vivo are unknown, several possibilities exist. The pattern of immunohistochemical staining in Figure 6D suggests that NTPDase1 was predominantly elevated in endothelial cells, with light staining on nerves and glial cells, consistent with findings in brain and retina,42,48–50 although precise cellular identification is not possible in sections in the absence of costaining. We have also demonstrated that isolated RGCs23 and optic nerve head astrocytes25 release ATP upon mechanical strain; it is thus likely that multiple cell types release ATP in response to sustained elevation of IOP.
It is important to stress that the “retinal” material used in this study also includes material from the optic nerve head. In contrast, the rat material used for optic nerve immunoblots in Figure 2E, and the primate material used for immunohistochemistry in Figure 6D panels iii and iv, was taken from the optic nerve distal to the sclera. A convergence of forces make it likely that the optic nerve head experiences higher rates of mechanical strain than other regions in glaucoma,51 and thus the response at the nerve head might be even greater than found in the retina or optic nerve itself. Recent evidence demonstrates that isolated optic nerve head astrocytes release a substantial amount of ATP upon stretch or swelling,25 although confirmation in an intact model would strengthen this finding.
Physiological Implications
The role of increased extracellular ATP in chronic glaucoma likely will vary with the concentration, ocular location, and age of the animal. The stimulation of the P2X7 receptor by excessive extracellular ATP can raise calcium and kill RGCs in vivo and in vitro, and can activate inflammatory cytokines in other cells.27,33,28 As the enzyme NTPDase1 dephosphorylates extracellular ATP, upregulation of this enzyme will reduce the time available for ATP to act at P2 receptors and increase the rate of adenosine production.47 Adenosine in turn can stimulate both A1 and A3 receptors on retina ganglion cells.24,52 As these adenosine receptors hyperpolarize ganglion cells, decrease their calcium levels and protect the cells from excitotoxic damage, the rise in adenosine is likely to be beneficial.52–54 Thus, the overall effect of increased extracellular ATP in glaucoma will depend upon whether the extracellular conversion of ATP into adenosine can keep up with the release of ATP, in addition to the relative expression of protective or damaging receptors.
Given that pathophysiological changes develop in many forms of glaucoma over the course of several years,44–46 it is important to demonstrate that any putative mechanism linking elevation of IOP to disease also shows a sustained change. This study provides strong support that the changes in purinergic signaling are sustained throughout the period of IOP elevation. Purinergic signaling systems are complex, and the protective and detrimental aspects of enhanced extracellular ATP and NTPDase1 levels must be dissected out. However, this study emphasizes that the window of time available to correct aberrant purinergic pathways is sustained.
Supplementary Material
Acknowledgments
The authors thank Elizabeth A. Hennes-Beann, Julie A. Kiland, Ann O'Brien Jenkins, Carol A. Rasmussen, Tatyana Shekhterman, and May Zhang for help with this manuscript.
Portions of this work have been published previously as ARVO abstracts: Lu W, et al. IOVS 2007;48:ARVO E-Abstract 4804; Lu W, et al. IOVS 2008;49:ARVO E-Abstract 869; and Lu W, et al. IOVS 2009;49:ARVO E-Abstract 114.
Supported by grants from the National Institutes of Health (NIH; Bethesda, Maryland, United States) EY015537 and EY013434 (CHM), EY10009 (AML), EY10564 (VCS), EY022077 (GSZ), EY010145 (JCM and ECJ) and EY016866 (ECJ and JCM), EY02698 and EY016665 (PLK), University of Pennsylvania (Philadelphia, Pennsylvania, United States) Vision Research Core Grant EY001583, the Jody Sack Fund (AML; Great Neck, New York, United States), Research to Prevent Blindness (AML, JCM, and ECJ; New York, New York, United States), the Paul and Evanina Bell Mackall Foundation Trust (AML; Chicago, IL, USA), and the Canadian Institutes of Health Research (CIHR; MOP-93683; JS; Ottawa, Ontario, Canada). VCS is an Investigator of the Howard Hughes Medical Institute (Chevy Chase, MD, USA). JS is the recipient of a “Chercheur National” Scholarship award from the Fonds de Recherche du Québec – Santé (FRQS, Montreal, PQ, Canada).
Disclosure: W. Lu, None; H. Hu, None; J. Sévigny, None; B.T. Gabelt, None; P.L. Kaufman, None; E.C. Johnson, None; J.C. Morrison, None; G.S. Zode, None; V.C. Sheffield, None; X. Zhang, None; A.M. Laties, None; C.H. Mitchell, None
References
- 1. Quigley HA,, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Osborne NN. Pathogenesis of ganglion “cell death” in glaucoma and neuroprotection: focus on ganglion cell axonal mitochondria. Prog Brain Res. 2008; 173: 339–352. [DOI] [PubMed] [Google Scholar]
- 3. AGIS. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000; 130: 429–440. [DOI] [PubMed] [Google Scholar]
- 4. Broman AT,, Quigley HA,, West SK, et al. Estimating the rate of progressive visual field damage in those with open-angle glaucoma, from cross-sectional data. Invest Ophthalmol Vis Sci. 2008; 49: 66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wax MB,, Tezel G. Immunoregulation of retinal ganglion cell fate in glaucoma. Exp Eye Res. 2009; 88: 825–830. [DOI] [PubMed] [Google Scholar]
- 6. Burgoyne CF. A biomechanical paradigm for axonal insult within the optic nerve head in aging and glaucoma. Exp Eye Res. 2011; 93: 120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Almasieh M,, Wilson AM,, Morquette B. Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012; 31: 152–181. [DOI] [PubMed] [Google Scholar]
- 8. Calkins DJ. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog Retin Eye Res. 2012; 31: 702–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nickells RW,, Howell GR,, Soto I,, John SW. Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu Rev Neurosci. 2012; 35: 153–179. [DOI] [PubMed] [Google Scholar]
- 10. Rieck J. The pathogenesis of glaucoma in the interplay with the immune system. Invest Ophthalmol Vis Sci. 2013; 54: 2393–2409. [DOI] [PubMed] [Google Scholar]
- 11. Guo Y,, Johnson EC,, Cepurna WO,, Dyck JA,, Doser T,, Morrison JC. Early gene expression changes in the retinal ganglion cell layer of a rat glaucoma model. Invest Ophthalmol Vis Sci. 2011; 52: 1460–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaufman PL. Enhancing trabecular outflow by disrupting the actin cytoskeleton increasing uveoscleral outflow with prostaglandins, and understanding the pathophysiology of presbyopia interrogating Mother Nature: asking why, asking how, recognizing the signs, following the trail. Exp Eye Res. 2008; 86: 3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krizaj D,, Ryskamp DA,, Tian N,, et al. From mechanosensitivity to inflammatory responses: new players in the pathology of glaucoma. Curr Eye Res. 2014; 39: 105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat. 1999; 194: 335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mitchell CH. Release of ATP by a human retinal pigment epithelial cell line: potential for autocrine stimulation through subretinal space. J Physiol. 2001; 534: 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fitz JG. Regulation of cellular ATP release. Trans Am Clin Climatol Assoc. 2007; 118: 199–208. [PMC free article] [PubMed] [Google Scholar]
- 17. Li A,, Leung CT,, Peterson-Yantorno K,, Mitchell CH,, Civan MM. Pathways for ATP release by bovine ciliary epithelial cells the initial step in purinergic regulation of aqueous humor inflow. Am J Physiol Cell Physiol. 2010; 299: C1308–C1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grygorczyk R,, Furuya K,, Sokabe M. Imaging and characterization of stretch-induced ATP release from alveolar A549 cells. J Physiol. 2013; 591: 1195–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lazarowski ER,, Boucher RC,, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003; 64: 785–795. [DOI] [PubMed] [Google Scholar]
- 20. Zhang X,, Li A,, Ge J,, Reigada D,, Laties A,, Mitchell C. Acute increase of intraocular pressure releases ATP into the anterior chamber. Exp Eye Res. 2007; 85: 637–643. [DOI] [PubMed] [Google Scholar]
- 21. Reigada D,, Lu W,, Zhang M,, Mitchell CH. Elevated pressure triggers a physiological release of ATP from the retina: Possible role for pannexin hemichannels. Neuroscience. 2008; 157: 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Resta V,, Novelli E,, Vozzi G, et al. Acute retinal ganglion cell injury caused by intraocular pressure spikes is mediated by endogenous extracellular ATP. Eur J Neurosci. 2007; 25: 2741–2754. [DOI] [PubMed] [Google Scholar]
- 23. Xia J,, Lim JC,, Lu W,, et al. Neurons respond directly to mechanical deformation with pannexin-mediated ATP release and autostimulation of P2X7 receptors. J Physiol. 2012; 590.10: 2285–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Newman EA. Glial cell inhibition of neurons by release of ATP. J Neurosci. 2003; 23: 1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beckel JM,, Argall AJ,, Lim JC, et al. Mechanosensitive release of ATP through pannexin channels and mechanosensitive upregulation of pannexin channels in optic nerve head astrocytes: a mechanism for purinergic involvement in chronic strain. Glia. 2014; 62: 1486–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li A,, Zhang X,, Zheng D,, Ge J,, Laties AM,, Mitchell CH. Sustained elevation of extracellular ATP in aqueous humor from humans with primary chronic angle-closure glaucoma. Exp Eye Res. 2011; 93: 528–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang DY,, Ray A,, Rodgers K, et al. Global gene expression changes in rat retinal ganglion cells in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010; 51: 4084–4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci. 2007; 28: 465–472. [DOI] [PubMed] [Google Scholar]
- 29. Morrison JC,, Moore CG,, Deppmeier LM,, Gold BG,, Meshul CK,, Johnson EC. A rat model of chronic pressure-induced optic nerve damage. Exp Eye Res. 1997; 64: 85–96. [DOI] [PubMed] [Google Scholar]
- 30. Zode GS,, Kuehn MH,, Nishimura DY, et al. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J Clin Invest. 2011; 121: 3542–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lam DY,, Kaufman PL,, Gabelt BT,, To EC,, Matsubara JA. Neurochemical correlates of cortical plasticity after unilateral elevated intraocular pressure in a primate model of glaucoma. Invest Ophthalmol Vis Sci. 2003; 44: 2573–2581. [DOI] [PubMed] [Google Scholar]
- 32. Wamsley S,, Gabelt BT,, Dahl DB, et al. Vitreous glutamate concentration and axon loss in monkeys with experimental glaucoma. Arch Ophthalmol. 2005; 123: 64–70. [DOI] [PubMed] [Google Scholar]
- 33. Zhang X,, Zhang M,, Laties AM,, Mitchell CH. Stimulation of P2X7 receptors elevates Ca2+ and kills retinal ganglion cells. Invest Ophthalmol Vis Sci. 2005; 46: 2183–2191. [DOI] [PubMed] [Google Scholar]
- 34. Reigada D,, Lu W,, Zhang X, et al. Degradation of extracellular ATP by the retinal pigment epithelium. Am J Physiol Cell Physiol. 2005; 289: C617–C624. [DOI] [PubMed] [Google Scholar]
- 35. Lu W,, Reigada D,, Sevigny J,, Mitchell CH. Stimulation of the P2Y1 receptor up-regulates nucleoside-triphosphate diphosphohydrolase-1 in human retinal pigment epithelial cells. J Pharmacol Exp Ther. 2007; 323: 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fausther M,, Lecka J,, Kukulski F, et al. Cloning, purification, and identification of the liver canalicular ecto-ATPase as NTPDase8. Am J Physiol Gastrointest Liver Physiol. 2007; 292: G785–G795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Enjyoji K,, Sevigny J,, Lin Y,, et al. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999; 5: 1010–1017. [DOI] [PubMed] [Google Scholar]
- 38. Smith TM,, Kirley TL. Glycosylation is essential for functional expression of a human brain ecto-apyrase. Biochemistry. 1999; 38: 1509–1516. [DOI] [PubMed] [Google Scholar]
- 39. Levesque SA,, Kukulski F,, Enjyoji K,, Robson SC,, Sevigny J. NTPDase1 governs P2X7-dependent functions in murine macrophages. Eur J Immunol. 2010; 40: 1473–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schulte am Esch J II,, Sevigny J,, Kaczmarek E, et al. Structural elements and limited proteolysis of CD39 influence ATP diphosphohydrolase activity. Biochemistry. 1999; 38: 2248–2258. [DOI] [PubMed] [Google Scholar]
- 41. Kaczmarek E,, Koziak K,, Sevigny J,, et al. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem. 1996; 271: 33116–33122. [DOI] [PubMed] [Google Scholar]
- 42. Wurm A,, Iandiev I,, Hollborn M, et al. Purinergic receptor activation inhibits osmotic glial cell swelling in the diabetic rat retina. Exp Eye Res. 2008; 87: 385–393. [DOI] [PubMed] [Google Scholar]
- 43. Zhang X,, Li A,, Ge J,, Reigada D,, Laties AM,, Mitchell CH. Acute increase of intraocular pressure releases ATP into the anterior chamber. Exp Eye Res. 2007; 85: 637–643. [DOI] [PubMed] [Google Scholar]
- 44. De Moraes CG,, Demirel S,, Gardiner SK, et al. Effect of treatment on the rate of visual field change in the ocular hypertension treatment study observation group. Invest Ophthalmol Vis Sci. 2012; 53: 1704–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. De Moraes CG,, Liebmann JM,, Liebmann CA,, Susanna R, Jr, Tello C,, Ritch R. Visual field progression outcomes in glaucoma subtypes. Acta Ophthalmol. 2013; 91: 288–293. [DOI] [PubMed] [Google Scholar]
- 46. Kim KE,, Jeoung JW,, Kim DM,, Ahn SJ,, Park KH,, Kim SH. Long-term follow-up in preperimetric open-angle glaucoma: progression rates and associated factors. Am J Ophthalmol. 2015; 159: 160–168, e161–e162. [DOI] [PubMed] [Google Scholar]
- 47. Robson SC,, Sevigny J,, Zimmermann H. The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal. 2006; 2: 409–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Braun N,, Sevigny J,, Robson SC, et al. Assignment of ecto-nucleoside triphosphate diphosphohydrolase-1/cd39 expression to microglia and vasculature of the brain. Eur J Neurosci. 2000; 12: 4357–4366. [PubMed] [Google Scholar]
- 49. Iandiev I,, Wurm A,, Pannicke T,, et al. Ectonucleotidases in Muller glial cells of the rodent retina: Involvement in inhibition of osmotic cell swelling. Purinergic Signal. 2007; 3: 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ricatti MJ,, Alfie LD,, Lavoie EG,, Sevigny J,, Schwarzbaum PJ,, Faillace MP. Immunocytochemical localization of NTPDases1 and 2 in the neural retina of mouse and zebrafish. Synapse. 2009; 63: 291–307. [DOI] [PubMed] [Google Scholar]
- 51. Sigal IA,, Ethier CR. Biomechanics of the optic nerve head. Exp Eye Res. 2009; 88: 799–807. [DOI] [PubMed] [Google Scholar]
- 52. Zhang X,, Zhang M,, Laties AM,, Mitchell CH. Balance of purines may determine life or death of retinal ganglion cells as A3 adenosine receptors prevent loss following P2X7 receptor stimulation. J Neurochem. 2006; 98: 566–575. [DOI] [PubMed] [Google Scholar]
- 53. Clark BD,, Kurth-Nelson ZL,, Newman EA. Adenosine-evoked hyperpolarization of retinal ganglion cells is mediated by G-protein-coupled inwardly rectifying K+ and small conductance Ca2+-activated K+ channel activation. J Neurosci. 2009; 29: 11237–11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang M,, Hu H,, Zhang X,, et al. The A3 adenosine receptor attenuates the calcium rise triggered by NMDA receptors in retinal ganglion cells. Neurochem Int. 2010; 56: 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.