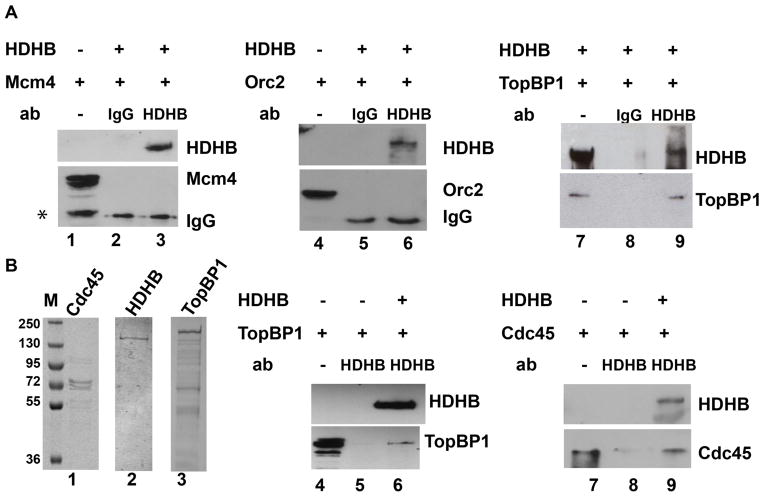
Figure 2. HDHB interacts with TopBP1 and Cdc45 in vitro.
(A) HDHB was immunoprecipitated from extracts of baculovirus-infected insect cells co-expressing HDHB and Mcm4, Orc2, or TopBP1 using non-immune rat IgG (lanes 2, 5, 8) or anti-HDHB 5c9 monoclonal antibody (lanes 3, 6, 9). Co-precipitated proteins were detected by western blot with the antibodies indicated to the right of each panel. Samples of extract from cells infected with baculoviruses expressing only Mcm4 (lane 1), only Orc2 (lane 4), or both HDHB and TopBP1 (lane 7) were electrophoresed in parallel. Asterisk: partially degraded Mcm4 (lane 1). (B) Purified bacterially expressed Cdc45 (lane 1), purified baculovirus-expressed HDHB (lane 2) and TopBP1 (lane 3) were visualized by SDS-PAGE and Coomassie staining. Protein A-agarose beads pre-bound to polyclonal HDHB antibody were incubated with purified TopBP1 or Cdc45 in the absence (lanes 5, 8) or presence of purified HDHB (lanes 6, 9). Samples (20%) of TopBP1 and Cdc45 input are shown in lanes 4 and 7. Precipitated proteins were analyzed by SDS-PAGE and western blot with the indicated antibodies.