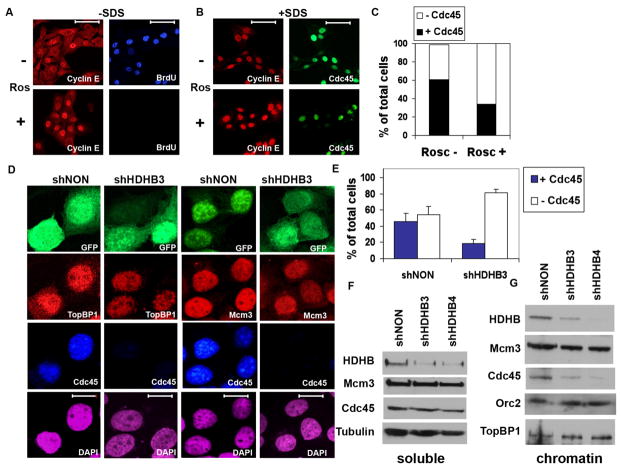
Figure 4. Cdc45 chromatin loading is reduced in HDHB-depleted cells.
(A) U2OS cells cultured with (+) or without (−) 50 μM roscovitine (Ros) as indicated were incubated with BrdU for 30 min, fixed and permeabilized using the standard protocol (−SDS), and stained with anti-BrdU and anti-cyclin E for immunofluorescence microscopy. Scale bar, 70 μm. (B) U2OS cells cultured + or − roscovitine as indicated (+SDS) were fixed with 1 % formaldehyde for 10 min, extracted with 0.1% Triton X-100 and 0.02% SDS [49,59], and stained with anti-cyclin E and anti-Cdc45 for immunofluorescence microscopy. (C) The fraction of Cdc45-positive nuclei in cultures treated + or − roscovitine and stained as in (B) was determined by counting 100 DAPI-stained cells each culture. (D, E) U2OS cells co-expressing GFP and shNON or shHDHB3 were fixed and permeabilized as in (B), and stained with antibodies against Mcm3, TopBP1, and Cdc45 as indicated. Nuclei were stained with DAPI. Scale bar, 15 μm. (E) The fraction of Cdc45-positive cells expressing shNON (n=190) or shHDHB3 (n=183) was determined in two independent experiments. Brackets indicate standard deviation. (F, G) Cells expressing control (shNON) or HDHB shRNA (shHDHB3 and shHDHB4) were fractionated into soluble (F) and chromatin (G) extracts, and analyzed by western blot with the indicated antibodies.