Figure 3. The effect of Clec16a silencing is not T cell-intrinsic.
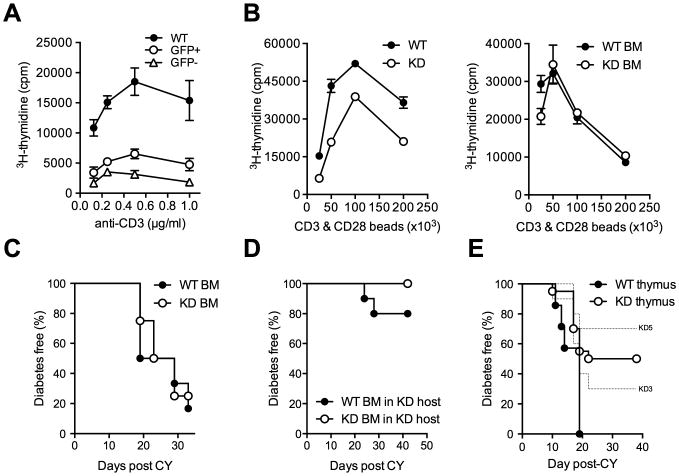
(A) Proliferation of GFP+ and GFP- Clec16a KD and WT CD4+ T cells stimulated with anti-CD3 antibody. (B) Proliferation of FACS-sorted CD4+ T cells from WT or KD mice (left panel) or from WT bone-marrow (BM) chimeric mice reconstituted with WT or KD BM (right panel) stimulated with anti-CD3/anti-CD28 coated beads. (C) CY diabetes in WT BM chimeric mice reconstituted with WT (n = 6) or KD (n = 4) BM. (D) CY diabetes in Clec16a KD BM chimeric mice reconstituted with WT (n = 10) or KD (n = 10) BM, P = 0.14. (E) Diabetes frequency in NOD mice thymectomized at 4 weeks of age and subsequently transplanted with fetal thymus (E14) from Clec16a KD (KD3 n = 10, KD5 n = 10) or WT (n= 7) embryos in conjunction with WT bone-marrow after irradiation, WT vs. KD - P = 0.0132. No difference was observed in either the size of thymuses post-transplantation or the frequency of T cells in transplanted mice at the time of diabetes onset (data not shown).
