SUMMARY
Obstructive sleep apnea (OSA) leads to recurrent arousals from sleep, oxygen desaturations, daytime sleepiness and fatigue. This can have an adverse impact on quality of life. The aims of this study were to compare: 1) quality of life between the general population and untreated OSA patients; and 2) changes of quality of life among OSA patients after two years of positive airway pressure (PAP) treatment between adherent patients and non-users. Propensity score methodologies were used in order to minimize selection bias and strengthen causal inferences. The enrolled OSA subjects (n=822) were newly diagnosed with moderate to severe OSA who were starting PAP treatment and the general population subjects (n=742) were randomly selected Icelanders. The Short Form 12 was used to measure quality of life. Untreated OSA patients had worse quality of life when compared to the general population. This effect remained significant after using propensity scores to select samples, balanced with regard to age, body mass index, gender, smoking, diabetes, hypertension and cardiovascular disease. We did not find significant overall differences between full and non-users of PAP in improvement of quality of life from baseline to follow up. However, there was a trend towards more improvement in physical quality of life for PAP adherent patients and the most obese subjects improved their physical quality of life more. The results suggest that co-morbidities of OSA such as obesity, insomnia and daytime sleepiness have a great effect on life qualities and need to be taken into account and addressed with additional interventions.
Keywords: Mental and physical health, improvement, treatment adherence, general population comparison
INTRODUCTION
Obstructive sleep apnea (OSA) is a disorder characterized by recurrent apneas and hypopneas during sleep associated with oxygen desaturation and arousals (Punjabi, 2008). OSA affects almost every system in the body, resulting in an increased incidence of hypertension, cardiovascular disease, stroke, pulmonary hypertension, cardiac arrhythmias, and systemic inflammation (McNicholas and Bonsigore, 2007). Sleep fragmentation due to OSA may also result in decreased energy, impaired cognition and altered mood (Soldatos and Paparrigopoulos, 2005) and OSA increases the risk of traffic and work accidents (Sassani et al., 2004). However, symptoms and the presence of co-morbidities vary among OSA patients (McNicholas and Bonsigore, 2007).
The daytime consequences of OSA are usually more important to the patient than the nocturnal events. Patients may be unaware of their snoring and breathing pauses during sleep, but acutely aware of the consequent daytime sleepiness, impaired work performance, irritability, and reduced participation in everyday activities (Chervin, 2000). As a result of these symptoms and functional impairments, OSA patients often report a poor quality of life in social, emotional and physical domains (Lacasse et al., 2000; Akashiba et al., 2002; Baldwin et al., 2001).
The most effective treatment of OSA is positive airway pressure (PAP), which has been shown to decrease sleepiness and improve neurocognitive function and vigilance (Gay et al., 2006). PAP treatment is mostly beneficial for patients who use PAP for at least 4 hours per night (Kohler et al., 2010). Studies have shown that untreated patients with severe OSA have reduced quality of life compared to normal controls (Bjornsdottir et al., 2012; Yang et al., 2000) and a few weeks of PAP treatment improves daytime function and quality of life (D’Ambrosio et al., 1999; Jenkinson et al., 1999; Ballester et al., 1999; Terri et al., 2012). A study by D’Ambrosio et al. (1999) found that eight weeks of PAP treatment by adherent patients improved aspects of quality of life related to vitality, social functioning, and mental health. In their study, the magnitude of improvement was, however, most strongly related to the degree of impairment in quality of life at baseline. In a randomized controlled trial by Jenkinson et al. (1999) improvements were seen in vitality scores and social function after one month of PAP treatment. Ballester et al. (1999) found positive effects on social isolation and energy subscales of quality of life after three months of PAP treatment. Furthermore, Weaver at al. (2012) found that 8 weeks of PAP treatment improved functional outcomes among sleepy patients with mild to moderate OSA.
Despite the evidence discussed above, there are few large studies with long-term follow-up periods that have assessed: 1) the difference in quality of life among OSA patients and the general population, and 2) how quality of life changes with long term PAP treatment between adherent patients and non-users. Given this, the aims of this study were two-fold. First, we compared health-related quality of life between the general population and a large group of patients with moderate to severe OSA prior to PAP treatment initiation. Second, we examined differences in the changes of quality of life after two years of PAP treatment between adherent patients and non-users.
MATERIALS AND METHODS
OSA cohort
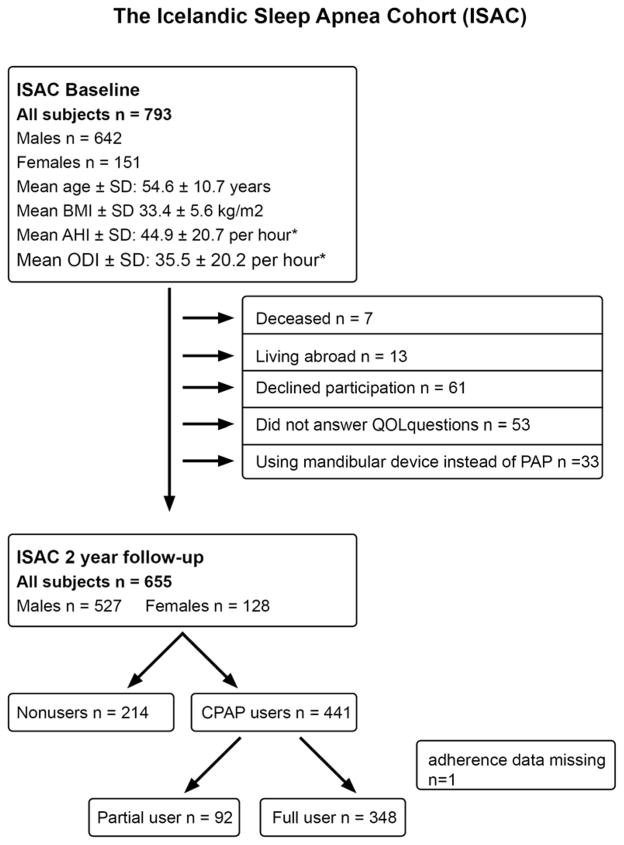
All patients diagnosed with moderate to severe OSA (apnea hypopnea index [AHI] ≥15 events/hr) who were referred to the Pulmonary Department, Landspitali – The National University Hospital of Iceland for treatment with PAP from September 2005 to December 2009 were invited to participate in the Icelandic Sleep Apnea Cohort (ISAC) study (Bjornsdottir et al., 2012; Arnardottir et al., 2012; Bjornsdottir et al, 2013). No other inclusion or exclusion criteria were used. Over 90% of eligible and approached subjects agreed to participate in the study, resulting in 822 subjects included in the prospective cohort at baseline. A total of 793 (96.5%) subjects (642 males and 151 females) had available information on quality of life. Two years after treatment initiation, participants were invited for a follow-up visit where treatment adherence to PAP was examined and baseline assessments were repeated. This follow-up was completed in 741 (90.1%) subjects from October 2007 to January 2012; 655 (88.4%) responded to questions regarding quality of life at follow-up and were not prescribed a mandibular advancement device instead of PAP treatment (Figure 1).
Figure 1.
Flow chart of the study population
General population cohort
The general population cohort was primarily invited to participate in the Burden of Obstructive Lung Diseases (BOLD) initiative; a multi-center international study aiming to estimate the burden of chronic obstructive pulmonary disorder worldwide (Buist et al., 2007). This was a random sample of Icelanders, ≥40 years living in the capital area of Reykjavik. Altogether 762 (404 males and 358 females) of the 939 eligible subjects (81.2%) responded. The mean age in this cohort was 57.0 ± 11.8 years and the mean BMI was 27.9 ± 4.9 kg/m2.
General health questionnaire
All participants were invited to the outpatient clinic at Landspitali - The National University Hospital of Iceland in Reykjavik. The study was approved by the National Bioethics Committee and the Data Protection Authority of Iceland, as well as the Institutional Review Board of the University of Pennsylvania. After written informed consent was obtained, participants answered standardized questionnaires about their health and sleep including questions about smoking and whether they had hypertension and/or diabetes (medical diagnosis and medication), or cardiovascular disease (CVD) which was defined as a medical diagnosis of coronary artery occlusion (myocardial infarction or heart attack), heart failure and/or stroke. Subjects in both cohorts listed their medication, which was subsequently coded according to the Anatomical Therapeutic Chemical (ATC) drug classification system (www.whocc.no/atcddd) and OSA subjects were asked whether they were taking medication to help them sleep.
Quality of life
Participants completed the Short Form 12 (SF-12) questionnaire to assess quality of life. Two summary component scores are derived from the SF-12, the physical component score (PCS) and mental component score (MCS). These scores range from 0–100, where a zero score indicates the lowest life quality and 100 indicates the highest life quality (Ware et al., 1996). The SF-12 is derived from the SF-36 and has been widely used and demonstrated to be reliable and valid in assessing quality of life in large group comparisons (Gandek et al., 1998).
Excessive daytime sleepiness
Daytime sleepiness was evaluated using the Epworth Sleepiness Scale (ESS), a brief questionnaire that measures daytime sleepiness. Participants with ESS score ≥10 were considered to have excessive daytime sleepiness (Johns, 1992).
Symptoms of sleep disorders
Sleep symptoms were assessed using the Basic Nordic Sleep Questionnaire, which includes questions on sleep quality, insomnia symptoms, snoring, nocturnal sweating, gastroesophageal reflux (GER) and daytime sleepiness (Partinen and Gislason, 1995). Three subtypes of insomnia symptoms were defined; difficulty initiating sleep (initial insomnia), difficulty maintaining sleep (middle insomnia) and early morning awakenings (late insomnia), for more details see previous publication (Bjornsdottir et al., 2013). However, the questionnaire for the general population only included questions on two of those subtypes (initial and middle insomnia). Answers were rated on a five point scale: never/almost never (1); less than once a week (2); once or twice a week (3); three to five times a week (4); every day or almost every day of the week (5).
Sleep recording in ISAC cohort
Prior to referral for PAP treatment, all OSA patients had a sleep study with an Embletta type 3 portable monitor or an Embla 12 channel system (Natus Medical Inc., Ontario, Canada) or a T3 device (Nox Medical, Reykjavik, Iceland). The same signals were recorded on all studies; nasal airflow by cannula, oxygen saturation, heart rate, respiratory movements by respiratory inductance plethysmography (RIP) belts, body position and activity by accelerometer. Trained sleep technologists scored all sleep studies and the studies had to have ≥4 hours of a scorable O2 saturation signal. Scoring of a hypopnea required a ≥ 30% decrease in airflow for ≥ 10 sec with ≥ 4% oxygen desaturation or ≥ 50% decrease in airflow for ≥ 10 sec with a sudden increase in flow at the end of the event. Scoring of an apnea required ≥ 80% decrease in flow for ≥ 10 sec. The AHI was calculated as the mean number of apneas and hypopneas per hour of recording (excluding upright time). The oxygen desaturation index (ODI) was calculated as the number of transient drops in oxygen saturation ≥4% per hour of recording. For further details, see our previous publications (Bjornsdottir et al., 2013; Arnardottir et al., 2013).
PAP use
All patients prescribed PAP received care at the Department of Respiratory Medicine and Sleep, Landspitali University Hospital. Patients on PAP had direct access to the outpatient clinic where trained staff helped them to find the type of device and settings they needed.
PAP adherence at the 2-year follow-up was estimated based on downloads of usage in the previous 4 weeks from memory cards (objective data), if available, from ResMed S8 machines (ResMed Corp. San Diego, CA, USA). Some subjects had older PAP devices that did not allow for this type of download. Self-report data from all subjects (subjective data) was collected at the follow-up, based on three multiple-choice questions about average PAP use. Self reported data had 98.6% sensitivity and 45.1% specificity in distinguishing full users from partial users. For further details, see our previous publications (Bjornsdottir et al., 2013; Arnardottir et al., 2013).
Participants who used PAP for ≥20 days and ≥4 hrs/day on average for the previous four weeks based on objective data or ≥5 nights/week for ≥60% of the night by questionnaire were considered full users. PAP users not meeting criteria for full users were classified as partial users; these patients were excluded from the propensity score sample estimating the effect of PAP treatment on changes in quality of life to allow propensity score matching between the 2 primary groups of interest — full users and non-users. Non-users were defined as those who had returned their PAP device within one year of therapy initiation and did not undergo upper airway surgery and were not using mandibular device.
Statistical analyses
All statistics were calculated with STATA, version 11.0 for Windows (Stata Corporation, College Station, TX) or SAS, Version 9.3 (SAS Institute, Cary, NC). For bivariate analysis, the chi-square test and t-test were used for nominal and continuous variables respectively. Linear regression was used in adjusted analyses and results are presented as adjusted β-estimates and 95% confidence intervals or adjusted least squares mean estimates and standard errors.
Propensity score sub-classification analyses
Sub-classification using propensity score (PS) quintiles, following an established sequential heuristic described in detail by Maislin and Rubin (2010), was used in two separate analyses: 1) to obtain a comparable sample of OSA patients and subjects from the general population with respect to relevant covariates; and 2) to minimize selection bias due to measured covariate imbalance in our non-randomized treatment group comparison between PAP adherent patients and non-users, thereby allowing for causal inference (Keenan et al., 2014). The importance of using PS and related methodologies within the context of observational studies has been highlighted by a recent working group from the National Heart, Lung and Blood Institute (Lieu et al., 2011). For further details, see supplement.
In order to obtain a comparable sample of OSA patients and subjects from the general population, we first restricted both populations to participants aged 40–75 years and with BMIs between 25–40 kg/m2, based on the obvious distributional differences in age and BMI between the two samples. This restriction resulted in an initial sample of 611 (74.3%) OSA patients and 471 (61.7%) general population subjects. Within this restricted sample, we used sub-classification by PS quintiles to further match samples on relevant covariates, including age, gender, BMI, smoking status, hypertension, cardiovascular disease, and diabetes. The PS heuristic identified 494 (80.9%) OSA patients and 418 (88.7%) general population participants from this restricted sample that were included in the final PS designed sample (referred to as the ‘OSA-general population PS Sample’).
Within the OSA cohort only, we then used PS sub-classification to construct a sample of PAP full and non-users in which to obtain an unbiased assessment of the effect of PAP adherence on changes in quality of life. Full and non-users were balanced within subclass with respect to relevant measured covariates at baseline, including: age, gender, BMI, smoking status, hypertension, cardiovascular disease, diabetes, insomnia symptoms (early, middle and late), sleep medication use, antidepressant use, ESS, OSA severity (AHI, ODI, SaO2 nadir, and hypoxia time [% of sleep time with SaO2 below 90%) and baseline levels of PCS and MCS. Of the 348 full and 214 non-users, a total of 308 (89%) and 200 (93%) were included in the PS designed sample (referred to as the ‘PAP Treatment PS Sample’). Comparisons of subjects included and excluded from the PS sample are presented in the online supplement.
RESULTS
Results in all subjects
Baseline characteristics in the overall sample
Overall, OSA patients were slightly younger, with a higher BMI, more daytime sleepiness, as well as a higher prevalence of hypertension, diabetes and middle insomnia when compared to subjects from the general population (Table 1). OSA subjects were also more likely to be males, have a smoking history and reported significantly lower mental and physical quality of life. Both MCS and PCS remained significantly lower among OSA patients after adjusting for age, BMI, gender, smoking and the co-morbidities listed in table 1; on average, OSA patients had PCS scores 9.48 points lower (95% CI: −10.53, −8.44; p<0.0001) and MCS scores 3.35 points lower (95% CI: −4.35, −2.35; p<0.0001) than the general population.
Table 1.
Baseline characteristics of all OSA patients and the general population cohort
| General population (n=762) | OSA (n=793) | P | |
|---|---|---|---|
| Age (years) | 57.0 ± 11.8 | 54.6 ± 10.7 | <0.0001 |
| BMI (kg/m2) | 27.9 ± 4.9 | 33.4 ± 5.6 | <0.0001 |
| Male (%) | 53.0 | 81.0 | <0.0001 |
| Smoking history | <0.0001 | ||
| Never smoker (%) | 39.2 | 27.3 | |
| Previous smoker (%) | 42.6 | 51.0 | |
| Current smoker (%) | 18.2 | 21.7 | |
| Hypertension (%) | 25.1 | 45.3 | <0.0001 |
| Cardiovascular disease (%) | 15.1 | 18.4 | 0.0781 |
| Diabetes (%) | 2.9 | 8.7 | <0.0001 |
| Epworth Sleepiness Scale (ESS) | 6.0 ± 3.9 | 11.7 ± 5.1 | <0.0001 |
| Early insomnia (%) | 14.1 | 15.3 | 0.5200 |
| Middle insomnia (%) | 17.3 | 34.8 | <0.0001 |
| Late insomnia (%) | — | 27.9 | — |
| Mental quality of life (MCS) | 51.4 ± 4.7 | 48.3 ± 10.9 | <0.0001 |
| Physical quality of life (PCS) | 50.9 ± 7.8 | 40.3 ± 10.9 | <0.0001 |
Values are given as mean ± standard deviation for continuous variables and percentages for nominal variables. Abbreviations: BMI, body mass index.
Determinants of quality of life in OSA patients and controls
We ran regression analyses within the overall OSA and general population cohorts to assess potential determinants of physical and mental quality of life. PCS was lower for those with higher BMI, both for OSA patients and the general population. Furthermore, higher age, female gender, and cardiovascular disease were significantly associated with a lower PCS in both groups; diabetes was associated with worse PCS in controls. Lower MCS was associated with lower age, current smoking and hypertension among controls, and with lower age, current smoking, female gender, and lower BMI among OSA patients (Table 2).
Table 2.
Independent association with physical (PCS) and mental (MCS) component scores in the general population cohort and OSA patients.
| General population (n=762) | OSA patients (n=793) | |||
|---|---|---|---|---|
|
| ||||
| PCS | MCS | PCS | MCS | |
| Age (years) | −0.12 (−0.17, −0.07) | 0.09 (0.05, 0.12) | −0.16 (−0.23, −0.08) | 0.16 (0.07, 0.24) |
| Female gender | −2.21 (−3.25, −1.16) | −0.52 (−1.2, 0.16) | −5.17 (−6.99, −3.36) | −2.48 (−4.45, −0.51) |
| BMI (kg/m2) | −0.20 (−0.31, −0.10) | 0.04 (−0.03, 0.11) | −0.53 (−0.66, −0.40) | 0.15 (0.01, 0.29) |
| Smoking history | ||||
| Previous smoker | −1.05 (−2.19, 0.09) | −0.71 (−1.46, 0.03) | −1.4 (−3.07, 0.26) | −0.74 (−2.56, 1.07) |
| Current smokers | −2.77 (−4.26, −1.28) | −1.01 (−1.98, −0.04) | −2.62 (−4.61, −0.63) | −4.18 (−6.34, −2.01) |
| Hypertension | −0.62 (−1.93, 0.69) | −1.35 (−2.2, −0.49) | 0.25 (−1.31, 1.8) | −0.9 (−2.59, 0.79) |
| Cardiovascular disease | −3.54 (−5.20, −1.87) | 0.44 (−0.64, 1.52) | −5.77 (−7.67, −3.86) | −1.27 (−3.34, 0.79) |
| Diabetes | −6.97 (−10.18, −3.77) | 0.98 (−1.1, 3.06) | 0.00 (−2.59, 2.59) | 1.1 (−1.71, 3.91) |
Values are given as adjusted beta-values (95% CI); estimates are adjusted for all other variables in the table.
When examining sleep symptoms (Table 3), symptoms of initial and middle insomnia were both associated with lower MCS and PCS among general population subjects. Among OSA patients, we found no significant relationships between severity of OSA, based on AHI and ODI, and either PCS or MCS. Among OSA patients, initial insomnia was associated with lower PCS and MCS, and late insomnia was associated with lower MCS. More subjective sleepiness was associated with decreased quality of life among OSA patients; no such relationship was seen among controls. Sleep medication and antidepressant use was associated with poorer PCS and MCS in OSA subjects. These data were not available in the general population sample (Table 3).
Table 3.
Independent associations of sleep symptoms with physical (PCS) and mental (MCS) component scores in general population subjects and OSA patients.
| General population (n=762) | OSA patients (n=793) | |||
|---|---|---|---|---|
|
| ||||
| PCS | MCS | PCS | MCS | |
| Epworth sleepiness score | 0.06 (−0.08, 0.20) | −0.08 (−0.16, 0.01) | −0.36 (−0.50, −0.22) | −0.27 (−0.42, −0.13) |
| Difficulties falling asleep | −2.90 (−4.49, −1.32) | −1.94 (−2.94, −0.95) | −2.62 (−4.72, −0.51) | −2.98 (−5.19, −0.77) |
| Difficulties maintain sleep | −1.49 (−2.94, −0.03) | −1.04 (−1.96, −0.13) | −1.03 (−2.49, 0.43) | 0.32 (−1.21, 1.85) |
| Early morning awakenings | n/a | n/a | −0.26 (−1.89, 1.36) | −2.24 (−3.94, −0.54) |
| Sleep medication use | n/a | n/a | −2.75 (−5.14, −0.36) | −4.37 (−6.87, −1.87) |
| Antidepressant use | n/a | n/a | −3.88 (−5.79, −1.98) | −5.88 (−7.88, −3.88) |
Values are given as adjusted beta-values (95% CI). The beta estimates are adjusted for all variables in the table and for gender, age and BMI.
OSA and general population cohorts: Results in the PS sample
The covariate balance achieved by applying our PS methodology to the OSA patients and general population subjects is shown in a modified version of a Love plot (Ahmed et al., 2006). Prior to implementing the PS heuristic, there were significant differences between OSA patients and controls for BMI (p<0.0001), gender (p<0.0001), past smoking status (p=0.001), never smoker status (p<0.0001), hypertension (p<0.0001), diabetes (p=0.0001) and cardiovascular disease (p=0.010). However, after sub-classification and controlling for PS quintile, there were no differences between OSA cases and general population subjects (all p>0.686). See Figure 1 in supplement.
When comparing PCS and MCS within this PS designed sample of OSA cases and general population subjects (Table 4), OSA patients had significantly lower quality of life in both measures; with a PCS score 9.5 points lower than controls (p<0.0001) and an MCS score 3.0 points lower (p<0.0001).
Table 4.
Comparison of baseline quality of life measures in the OSA-general population propensity score sample
| LS Mean ± SE
|
Difference (95% CI) | p* | ||
|---|---|---|---|---|
| OSA patients | General population | |||
|
|
|
|
|
|
| Physical Quality of Life | 41.4 ± 0.45 | 50.9 ± 0.48 | −9.48 (−10.81, −8.14) | <0.0001 |
| Mental Quality of Life | 48.3 ± 0.40 | 51.3 ± 0.43 | −2.95 (−4.15, −1.75) | <0.0001 |
p-value adjusted for propensity score subclass
Change in quality of life among OSA patients in OSA-general population PS sample 2 yearsafter initiating PAP treatment
When looking at OSA patients in the OSA-general population PS sample, both PCS (mean ± SE change: 2.6 ± 0.4; p<0.0001) and MCS (1.9 ± 0.5; p<0.001) increased significantly two years after treatment initiation. Despite these significant increases, PCS values in OSA patients at the 2-year follow-up remained significantly lower than the values seen in the general population at baseline (mean ± SE after 2 years: 43.9 ± 0.5 vs. 50.9 ± 0.5, p<0.0001). MCS values were borderline non-significantly lower in the OSA group after 2 years of therapy than the general population at baseline (50.3 ± 0.40 vs. 51.3 ± 0.42, p=0.072). In the current PS sample, full PAP users had an average increase in BMI of 0.9 kg/m2, compared to a non-significant increase of 0.09 kg/m2 in non-users (p<0.001 comparing full vs. non-users). Within the overall observational sample, we observed a significant negative correlation (ρ=−0.18, p<0.0001) between BMI change and PCS change, controlling for baseline BMI and PCS; thus, patients with more weight loss had more positive PCS changes.
Positive airway pressure full vs. non-users: Results in the PAP treatment PS sample
Prior to implementing the PS heuristic, there were significant differences between full and non-users in terms of BMI (p=0.01), gender (p=0.04), hypertension (p=0.001), initial (p=0.004) and late (p=0.001) insomnia, sleep medication use (p=0.02) and all OSA severity measures (all p<0.0001). After sub-classification and controlling for PS quintile, there were no differences between full and non-users (all p>0.677). See Figure 2 in supplement.
In the PAP Treatment PS Sample, significant 2-year increases (p<0.05) in both quality of life measures were observed for full and non-users, separately. When comparing the 2-year changes in physical and mental quality of life between PAP groups, we found only a borderline difference between full and non-users for change in PCS (p=0.06) and no differences in the change in MCS (p=0.80). While we did not observe significant evidence for a PAP by BMI group interaction, we did find a significant difference for PCS when we restricted analysis to BMI>35 (p=0.02) such that subjects adherent to PAP had a significantly greater improvement in PCS than non-users. No differences in PAP full and non-users related to BMI groups were found for MCS (Table 5).
Table 5.
Comparison of Change in Quality of Life in PAP Treatment propensity score sample
| BMI Group*,† |
LS Mean±SE Change
|
p‡ | ||
|---|---|---|---|---|
| Adherent | Non-Users | |||
| Physical Quality of Life | Overall | 3.42 ± 0.53§ | 1.79 ± 0.66§ | 0.0631 |
| <30 | 2.91 ± 0.97§ | 1.73 ± 1.15 | 0.4511 | |
| 30–35 | 2.50 ± 0.89§ | 2.49 ± 1.08§ | 0.9959 | |
| ≥35 | 4.64 ± 0.88§ | 1.05 ± 1.22 | 0.0216 | |
|
| ||||
| Mental Quality of Life | Overall | 2.13 ± 0.54§ | 2.35 ± 0.68§ | 0.7988 |
| <30 | 2.60 ± 0.97§ | 4.06 ± 1.15§ | 0.3525 | |
| 30–35 | 0.98 ± 0.96 | 1.32 ± 1.17 | 0.8313 | |
| ≥35 | 2.80 ± 0.88§ | 1.96 ± 1.22 | 0.5892 | |
p-value from ANCOVA model, adjusted for PS subclass and baseline PCS or MCS;
within group estimate of quality of life change significantly (p<0.05) different from zero;
p-value for PAP x BMI group interaction: 0.5266 for PCS and 0.4579 for MCS
Given our previously reported co-morbidity of OSA and insomnia (Bjornsdottir et al., 2012; Bjornsdottir et al., 2013), we also examined the effect of PAP treatment within strata defined by baseline subjective sleepiness or insomnia We did not observe any significant difference in quality of life changes between PAP groups when comparing patients based on ESS at baseline. When looking within patients with and without insomnia at baseline, we observed a significant difference in PCS change between full users and PAP non-users for those who did not have initial (p=0.02), middle (p=0.01) or late (p=0.02) insomnia at baseline; full users had greater increases in PCS (Table 3 in supplement).
While we observed statistically significant results within these strata, we note that we did not observe significant interactions between PAP group and any of these cut points; thus, results should be considered suggestive and replicated within independent populations. Given the established relationship between quality of life and depression, we also examined the effect of PAP in patients stratified by antidepressant use at baseline was analyzed. We did not observe a significant interaction between PAP adherence and antidepressant medication use for either PCS (p=0.498) or MCS (p=0.327) and there were no significant differences between PAP groups in PCS or MCS change within strata (Table 4 in supplement).
DISCUSSION
The main findings of this study are that untreated OSA patients have impaired physical and mental quality of life when compared to a general population sample. This effect remains significant after using propensity scores to select a sample of OSA patients and a general population sample, balanced with regard to age, BMI, gender, smoking status, diabetes, hypertension and cardiovascular disease. A significant improvement after a 2-year follow-up was seen in all patients, but full users of PAP do not appear to improve their quality of life more than non-users.
Among OSA patients, age, gender, BMI, cardiovascular disease, sleepiness, initial insomnia, sleep medication and antidepressant were all significant determinants of decreased quality of life. The fact that quality of life was decreased among those who reported sleep medication and antidepressant use could reflect that those who have impaired physical and mental health are more likely to use these medications than those who are more healthy.
We did not find significant overall differences between full and non-users of PAP in improvement of physical and mental quality of life from baseline to follow up within our overall propensity score matched sample. However, we did observe a significantly larger improvement in PCS for adherent patients compared to non-users within the most obese patients (BMI>35); this stronger effect of PAP in the most obese has been seen previously for other outcomes (Pak et al., 2014). We also observed a significant effect of PAP on PCS in patients with no insomnia symptoms at baseline, although this result needs to be replicated within independent samples. Taken together, results suggest that PAP may have a significant impact on physical quality of life within specific subsets of OSA patients. No differences were found in mental quality of life in these subgroups.
Reduced quality of life among OSA patients
Our results show that OSA significantly impairs quality of life and even though we observed significant increases in quality of life after two-years, these patients still have lower life qualities, particularly physical quality of life, when compared to subjects from the general population. Others have reported similar associations of OSA and poor life quality (Lacasse et al., 2000; Akashiba et al., 2002; Baldwin et al., 2001). Finn et al. (1998) assessed self-reported general health status in 421 men and 316 women aged 30–60 years in a general community sample and reported that OSA was independently related to lower general health. The association remained significant after adjustment for age, gender, BMI, smoking status, alcohol use, and cardiovascular conditions.
In our study, sleep parameters, such as symptoms of insomnia, daytime sleepiness and sleep medication use, were more related to quality of life than OSA severity measured by apneas per hour of sleep or hypoxemia. Others have found similar results. For example, a population-based study by Baldwin et al. (2001) suggested that mild OSA was related to reduced vitality, while more severe OSA was more broadly associated with reduced quality of life. That study also indicated that subjective sleep symptoms (sleepiness and disturbed sleep) are widely associated with poor quality of life (Baldwin et al., 2001). A study by Silvia et al. (2009) found that changes in quality of life over a 5 year period were not related to changes in OSA severity, but rather to worsening of difficulties initiating and maintaining sleep, as well as daytime sleepiness. Akashiba et al., (2002) found that mood or depression has more effect on quality of life than OSA severity and excessive daytime sleepiness. Others have reported similar results regarding the association of depression and quality of life (Kawahara et al., 2005; Diamanti et al., 2013). Unfortunately, measures of depression were unavailable in our study, but we did ask about antidepressant use and found a strong relationship with worse quality of life. Furthermore, we controlled for insomnia symptoms in our analysis, which are highly correlated to symptoms of depression (Lustberg and Reynolds 2000).
The effect of PAP treatment
We did not find a statistically significant difference in the improvement in quality of life between full and non-users of PAP, with both groups showing significant improvements from baseline to follow up. However, we note that the p-value for a difference in the change in PCS between full and non-users was borderline significant (p=0.063), with full users showing a larger increase on average compared to non-users. Previous studies have not assessed the difference in improvement in quality of life between full and non-PAP users. Others have, however, reported improvement in quality of life among OSA patients who adhere to PAP treatment (Diamanti et al., 2013; Avlonitou et al., 2012). In the study by Diamanti et al. (2013), only patients with more than 5 hours of PAP use were included, and the study by Avlonitou et al. (2012) also excluded patients who were not adherent to PAP treatment. Furthermore, a study by D’Ambrosio et al., (1999) reporting positive effects of PAP treatment on quality of life, assessed change from baseline in a small sample of adherent patients (N=29) and the magnitude of improvement was most strongly related to the degree of impairment in quality of life at baseline. In order to assess whether PAP treatment had an effect on changes in quality of life independent of baseline levels, adherent patients and non-users were matched for baseline quality of life in our propensity score sample.
A study by Jenkinson el al. (1997) showed positive effects of 5–7 weeks of continuous PAP (CPAP) on quality of life, measured by the SF-36, with effect sizes in the Energy/Vitality dimension of 0.98, and 0.76 for the MCS and 0.57 for the PCS. They concluded that CPAP treatment returns OSA patients to a quality of life similar to the normal population, which we did not see for physical quality of life in the present study. The study by Jenkinson et al. (1997) did not assess differences in improvement between patients with different adherence to CPAP. Furthermore, Sanner et al. (2000) concluded that long term CPAP treatment had a positive effect on quality of life. They did not, however, find a significant correlation between CPAP use and change in the quality of life measures. These studies may therefore have methodological issues that impacted their results. If we had only assessed changes in quality of life among full users in our study, we would have observed similar associations as found in previous studies (D’Ambrosio et al., 1999; Diamanti et al., 2013, Avlonitou et al., 2012; Jenkinson et al., 1997; Sanner et al., 2000).
A randomized controlled study by Montserrat et al. (2001) of the effectiveness of CPAP treatment, using a sham CPAP as a placebo for the control group, found positive effects of CPAP on daytime sleepiness and vitality. In this study, there was, however, no difference found in quality of life scores, measured by the SF-36, between those who were treated with CPAP as compared to placebo. Similar results were found in another randomized controlled trial using sham CPAP, with no significant benefits of CPAP over placebo on quality of life measured by the SF-36 (Barnes et al., 2000). Jenkinson et al (1999) did, however, find a positive effect on quality on life when comparing therapeutic CPAP to sub therapeutic CPAP in a randomized study and Ballester et al. (1999) reported similar results when comparing conservative treatment (sleep hygiene and weight loss therapy) alone to conservative treatment plus CPAP.
In a meta-analysis conducted by Jing et al. (2008), it was concluded that PAP treatment does not improve general quality of life scores but does improve physical domains and vitality. It is possible that some subgroups of patients improve their quality of life on PAP, as we saw for obese subjects and those without insomnia.
Even though quality of life is significantly improved from baseline to follow up for all OSA patients (both PAP users and nonusers) in our study, these patients still have much poorer physical health than subjects from the general population even after 2 years of therapy. OSA patients have various co-morbidities, such as obesity, high blood pressure and disturbed sleep, and these symptoms are often still apparent even though the OSA is well treated. In our observational sample, full users showed a higher increase in BMI from baseline to follow-up as compared to non-users. This increase in BMI while on PAP is likely to limit the benefit that would have occurred had patients who were adherent to PAP not gained weight. This indicates a need for more personalized medicine including broader therapeutic interventions that target co-morbidities such as obesity and sleep difficulties. In our previous study (Bjornsdottir et al., 2013) we found that symptoms of initial insomnia tend to persist in spite of successful PAP treatment. Therefore, it may well be that some of these patients have another untreated disorder and/or health condition besides OSA that impacts their quality of life. The existence of these underlying comorbidities could help explain why quality of life is still significantly impaired, despite successful PAP treatment. Long term follow up studies are needed to explore how PAP treatment affects co-morbid symptoms related to quality of life and well-being of OSA patients. Particularly, there is a need for studies on more personalized treatment interventions for these patients, for example additional interventions for those suffering from co-morbid insomnia.
The major strengths of this study include the large clinical cohort of OSA patients, the comparison with a general population cohort using propensity matching and the comparison between OSA patients with different degree of PAP use, as well as the extensive two-year follow-up with a high response rate (>90%). Furthermore, this study included detailed questionnaire assessments of co-morbid conditions as well as sleep studies in all OSA subjects. The major limitations were that we did not have measures of depression, which is an important indicator of quality of life. We did, however, control for insomnia symptoms and the use of antidepressants. Although a majority of patients (75%) using PAP had objective adherence information, a subset of patients included in this manuscript were classified based on subjective data only. As described in previous publications (Bjornsdottir et al., 2013; Arnardottir et al., 2013), our subjective criteria has high sensitivity (98.6%) and moderate specificity (45.1%) for classifying full vs. partial users. This combination of high sensitivity and low specificity means that full users are likely to be correctly classified, but also that a proportion of patients meeting our subjective criteria of full usage are actually partial users. While not ideal, the misclassification of partial users as full users in this minority of patients is expected to bias us towards the null, i.e., against observing a strong PAP effect. Moreover, we observed no significant changes in our estimates or results when restricting to only the subset of PAP users that had objective data. Furthermore, we did not have short-term follow-up results to evaluate the acute effects of PAP treatment on mental and physical health and we used the SF-12 to measure quality of life whereas using SF-36 could have potentially provided better discrimination. PS methodologies create balance with respect to measured covariates included in the matching, however, we cannot rule out the potential for unknown or unrecognized confounding. Moreover, we note that in order to obtain a well-matched sample of OSA patients and general population subjects, we necessarily excluded patients with higher obesity and the most co-morbidities, while simultaneously including less healthy and more obese general population subjects. While this restriction may reduce the scope of our inference, we note that our sample is more inclusive of “real-world” patients than typical randomized trials. Finally, while we recognize that there is likely a subset of the general population with undiagnosed OSA, including these patients as controls is expected to bias our results towards the null, suggesting that the significant differences observed in this study may be even greater.
Taken together, the results of the current study show that OSA patients have impaired quality of life compared to the general population, and, although a significant improvement after a 2-year follow-up was seen in all patients, full users of PAP do not appear to significantly improve their quality of life more than non-users. Despite the lack of statistical significance, there was a trend towards more improvement in PCS for PAP adherent patients compared to non-users in the overall population. Moreover, we found significant differences in specific subsets, including the most obese and those without insomnia; full users in these subgroups showed more improvement in physical quality of life compared to non-users. Co-morbidities of OSA such as obesity, insomnia symptoms and daytime sleepiness have a great effect on quality of life and these factors need to be taken into account and addressed with additional treatment interventions.
Supplementary Material
Figure 1. Modified Love plot comparing OSA patients (n=494) and general population subjects (n=418) before and after PS matching
Figure 2. Modified Love Plot Comparing PAP Full (n=308) and Non-users (n=200).
Table 1: Comparison of patients included in and excluded from PS designed observational study
Table 2: Comparison of patients included in and excluded from PS designed observational study
Table 3: Comparison of change in quality of life between full and non-users in propensity score matched sample stratified by sleepiness and insomnia symptoms at baseline
Table 4: Association between PAP Adherence and PCS and MCS change, stratified by antidepressant medication use
Acknowledgments
We are grateful to Sigrun Gudmundsdottir, Lovisa Gudmundsdottir, Magdalena Osk Sigurgunnarsdottir, Oddny Fjola Larusdottir, Kristjan Andri Kristjansson, Björn Magnússon, Bethany Staley, Greg Maislin, Nick Jackson, and the other staff at the Sleep Centers of Landspitali – The National University Hospital of Iceland and the University of Pennsylvania who helped assemble and analyze the data. This work was supported by the NIH grant HL72067 for “A Family Linkage Study of Obstructive Sleep Apnea” and HL94307 for “Endophenotypes of Sleep Apnea and Role of Obesity”, the Eimskip Fund of the University of Iceland and the Landspitali University Hospital Research Fund.
Footnotes
Declaration of contribution:
Erla Bjornsdottir designed the study with Thorarinn Gislason, Bryndis Benediktsdottir, Erna Sif Arnardottir and Allan I Pack. Erla performed the literature search, statistical analyses with the help of Brendan Keenan and Crister Janson. Erla drafted the paper and participated in all revisions of the paper with the co-authors
Brendan Keenan performed the propensity matching analyses and participated in data interpretation and all revisions of the manuscript.
Bjorg Eysteinsdottir participated in the data collection, the literature review and in all revisions of the manuscript.
Erna Sif Arnardottir participated in the study design, data collection and all revisions of the manuscript.
Christer Janson participated in statistical analyses, data interpretation and all revisions of the manuscript.
Thorarinn Gislason co-supervised the work, designed the study and participated in data interpretation and all revisions of the manuscript
Jon F Sigurdsson participated in all revisions of the manuscript and co-supervised the work.
Sam T. Kuna participated in data interpretation and all revisions of the manuscript
Allan I Pack co-supervised the work, designed the study and participated in data interpretation and all revisions of the manuscript
Bryndis Benediktsdottir co-supervised the work, designed the study and participated in data interpretation and all revisions of the manuscript
Conflict of interest:
The authors declare no competing interests with regard to the submitted work. Dr. Arnardottir is a consultant for Nox Medical, Inc. Dr. Kuna receives grant support from Philips Respironics. Other authors do not report a conflict of interest.
References
- Ahmed A, Husain A, Love TE, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akashiba T, Kawahara S, Akahosni T, et al. Relationship between quality of life and mood or depression in patients with severe obstructive sleep apnea syndrome. Chest. 2002;122:861–865. doi: 10.1378/chest.122.3.861. [DOI] [PubMed] [Google Scholar]
- Arnardottir ES, Maislin G, Schwab RJ, et al. The interaction of obstructive sleep apnea and obesity on the inflammatory markers C-reactive protein and interleukin-6: the Icelandic Sleep Apnea Cohort. Sleep. 2012;35:921–932. doi: 10.5665/sleep.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnardottir ES, Janson C, Bjornsdotttir E, et al. Nocturnal sweating--a common symptom of obstructive sleep apnoea: the Icelandic sleep apnoea cohort. BMJ open. 2013:3. doi: 10.1136/bmjopen-2013-002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avlonitou E, Kapsimalis F, Varouchakis G, Vardavas C, Behrakis P. Adherence to CPAP therapy improves quality of life and reduces symptoms among obstructive sleep apnea syndrome patients. Sleep Breath. 2012;16:563–569. doi: 10.1007/s11325-011-0543-8. [DOI] [PubMed] [Google Scholar]
- Baldwin CM, Griffith KA, Nieto FJ, O’connor GT, Walsleben JA, Redline S. The association of sleep disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health Study. Sleep. 2001;24:96–105. doi: 10.1093/sleep/24.1.96. [DOI] [PubMed] [Google Scholar]
- Ballester E, Badia JR, Hernández L, et al. Evidence of the effectiveness of continuous positive airway pressure in the treatment of sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:495–501. doi: 10.1164/ajrccm.159.2.9804061. [DOI] [PubMed] [Google Scholar]
- Barnes M, Houston D, Worsnop C, et al. A randomized controlled trial of continuous positive airway pressure in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:773–780. doi: 10.1164/ajrccm.165.6.2003166. [DOI] [PubMed] [Google Scholar]
- Bjornsdottir E, Janson C, Gislason T, et al. Insomnia in untreated sleep apnea patients compared to controls. J Sleep Res. 2012;21:131–8. doi: 10.1111/j.1365-2869.2011.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsdottir E, Janson C, Sigurdsson J, et al. Symptoms of insomnia among OSA patients before and after 2 years of PAP treatment. Sleep. 2013;36:1901–1909. doi: 10.5665/sleep.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370:741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- Chervin RD. Sleepiness, fatigue, tiredness and lack of energy in obstructive sleep apnea. Chest. 2000;118:372–379. doi: 10.1378/chest.118.2.372. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio C, Bowman T, Mohsenin V. Quality of life in patients with obstructive sleep apnea: Effect of nasal continuous positive airway pressure – A prospective study. Chest. 1999;115:123–129. doi: 10.1378/chest.115.1.123. [DOI] [PubMed] [Google Scholar]
- Diamanti C, Manali E, Ginieri-Coccossis M, et al. Depression, physical activity, energy consumption, and quality of life in OSA patients before and after CPAP treatment. Sleep Breath. 2013;17:1159–1168. doi: 10.1007/s11325-013-0815-6. [DOI] [PubMed] [Google Scholar]
- Gay P, Weaver T, Loube D, Iber C. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults: A review by the positive airway pressure task force of the standards of practice committee of the American academy of sleep medicine. Sleep. 2006;29:381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- Finn L, Young T, Palta M, Fryback DG. Sleep-disordered breathing and self-reported general health status in the Wisconsin Sleep Cohort Study. Sleep. 1998;21:701–708. [PubMed] [Google Scholar]
- Gandek B, Ware JE, Aaronson NK, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. J Clin Epidemiol. 1998;51:1171–1178. doi: 10.1016/s0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- Jenkinson C, Stradling J, Petersen S. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. J Sleep Res. 1997;6:199–204. doi: 10.1046/j.1365-2869.1997.00043.x. [DOI] [PubMed] [Google Scholar]
- Jenkinson C, Davies RJ, Mullins R, et al. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet. 1999;353:2100–2105. doi: 10.1016/S0140-6736(98)10532-9. [DOI] [PubMed] [Google Scholar]
- Jing J, Huang T, Cui W, Shen H. Effect on quality of life of continuous positive airway pressure in patients with obstructive sleep apnea syndrome: a meta-analysis. Lung. 2008;186:131–144. doi: 10.1007/s00408-008-9079-5. [DOI] [PubMed] [Google Scholar]
- Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–381. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- Kawahara S, Akashiba T, Akahoshi T, Horie T. Nasal CPAP improves the quality of Life and lessens the depressive symptoms in patients with obstructive sleep apnea syndrome. Intern Med. 2005;44:422–427. doi: 10.2169/internalmedicine.44.422. [DOI] [PubMed] [Google Scholar]
- Keenan BT, Maislin G, Sunwoo BY, et al. Obstructive Sleep Apnea Treatment and Fasting Lipids: A Comparative Effectiveness Study. Eur Respir J. 2014 May 15; doi: 10.1183/09031936.00043614. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler M, Smith D, Tippett V, Strading JR. Predictors of long-term compliance with continuous positive airway pressure. Thorax. 2010;65:829–832. doi: 10.1136/thx.2010.135848. [DOI] [PubMed] [Google Scholar]
- Lacasse Y, Godbout C, Sériés F. Health-related quality of life in obstructive sleep apnoea. Eur Respir J. 2000;19:499–503. doi: 10.1183/09031936.02.00216902. [DOI] [PubMed] [Google Scholar]
- Lieu TA, Au D, Krishnan JA, et al. Comparative effectiveness research in lung diseases and sleep disorders recommendations from the National Heart, Lung, and Blood Institute Workshop. Am J Respir Crit Care Med. 2011;184:848–856. doi: 10.1164/rccm.201104-0634WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustberg L, Reynolds CF., III Depression and insomnia: questions of cause and effect. Sleep Med Rev. 2000;4:253–262. doi: 10.1053/smrv.1999.0075. [DOI] [PubMed] [Google Scholar]
- Maislin G, Rubin DB. Design of non-randomized medical device trials based on sub-classification using propensity score quintiles. JSM Proceedings, Biopharmaceutical Section; Alexandria, VA: American Statistical Association; 2010. pp. 2182–2196. [Google Scholar]
- McNicholas WT, Bonsigore MR. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29:156–78. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- Montserrat JM, Ferrer M, Hernandez L, et al. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care Med. 2001;164:608–613. doi: 10.1164/ajrccm.164.4.2006034. [DOI] [PubMed] [Google Scholar]
- Pak VM, Keenan BT, Jackson N, et al. Adhesion molecule increases in sleep apnea: beneficial effect of positive airway pressure and moderation by obesity. Int J Obes. 2014 doi: 10.1038/ijo.2014.123. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partinen M, Gislason T. Basic Nordic Sleep Questionnaire (BNSQ): a quantitated measure of subjective sleep complaints. J Sleep Res. 1995;4:150–155. doi: 10.1111/j.1365-2869.1995.tb00205.x. [DOI] [PubMed] [Google Scholar]
- Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanner BM, Klewer J, Trumm A, Randerath W, Kreuzer I, Zidek W. Long-term treatment with continuous positive airway pressure improves quality of life in obstructive sleep apnoea syndrome. Eur Respir J. 2000;16:118–122. doi: 10.1034/j.1399-3003.2000.16a21.x. [DOI] [PubMed] [Google Scholar]
- Sassani A, Findley LJ, Kryger M, Goldlust E, George C, Davidson TM. Reducing motor-vehicle collisions, costs, and fatalities by treating obstructive sleep apnea syndrome. Sleep. 2004;27:453–458. doi: 10.1093/sleep/27.3.453. [DOI] [PubMed] [Google Scholar]
- Silva GE, An MW, Goodwin JL, et al. Longitudinal evaluation of sleep disordered breathing and sleep symptoms with change in quality of life: The Sleep Heart Health Study. Sleep. 2009;32:1049–1057. doi: 10.1093/sleep/32.8.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldatos CR, Paparrigopoulos TJ. Sleep physiology and pathology: pertinence to psychiatry. Int Rev Psychiatry. 2005;17:213–228. doi: 10.1080/09540260500104565. [DOI] [PubMed] [Google Scholar]
- Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Weaver TE, Mancini C, Maislin G, et al. Continuous positive airway pressure treatment of sleepy patients with milder obstructive sleep apnea. Am J Respir Crit Care Med. 2012;186:677–683. doi: 10.1164/rccm.201202-0200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang EH, Hla KM, McHorney CA, Havighurst T, Badr MS, Weber S. Sleep apnea and quality of life. Sleep. 2000;23:535–541. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1. Modified Love plot comparing OSA patients (n=494) and general population subjects (n=418) before and after PS matching
Figure 2. Modified Love Plot Comparing PAP Full (n=308) and Non-users (n=200).
Table 1: Comparison of patients included in and excluded from PS designed observational study
Table 2: Comparison of patients included in and excluded from PS designed observational study
Table 3: Comparison of change in quality of life between full and non-users in propensity score matched sample stratified by sleepiness and insomnia symptoms at baseline
Table 4: Association between PAP Adherence and PCS and MCS change, stratified by antidepressant medication use