Abstract
Accumulating evidence suggests that the mechanical and biochemical signals originating from cell-cell adhesion are critical for stem cell lineage specification. In this review, we focus on the role of cadherin mediated signaling in development and stem cell differentiation, with emphasis on two well-known cadherins, cadherin-2 (CDH2) (N-cadherin) and cadherin-11 (CDH11) (OB-cadherin). We summarize the existing knowledge regarding the role of CDH2 and CDH11 during development and differentiation in vivo and in vitro. We also discuss engineering strategies to control stem cell fate decisions by fine-tuning the extent of cell-cell adhesion through surface chemistry and microtopology. These studies may be greatly facilitated by novel strategies that enable monitoring of stem cell specification in real time. We expect that better understanding of how intercellular adhesion signaling affects lineage specification may impact biomaterial and scaffold design to control stem cell fate decisions in three-dimensional context with potential implications for tissue engineering and regenerative medicine.
Keywords: CDH2, CDH11, adherens junctions, differentiation, mesenchymal stem cells, microfabrication, micropatterning
1. Introduction
Intercellular adhesion plays important role in tissue architecture and morphogenesis by controlling the assembly of individual cells into the three-dimensional tissues[1]. Cell-cell or cell-matrix interactions are mediated by cell adhesion molecules (CAMs) including cadherins, integrins, selectins and immunoglobulin-like CAMs, and regulate multiple aspects of cellular behavior including proliferation, differentiation, apoptosis, cell polarity[1, 2], embryonic stem cell self-renewal and differentiation[3] and overall, the maintenance of tissue integrity [4].
Cadherins represent one class of CAMs that mediate Ca2+ dependent homophilic interactions between cells, through formation of intercellular connections or otherwise known as adherens junctions (AJs). The most well studied cadherins are the classical vertebrate cadherins that have been named based on the tissue in which they are expressed. Neuronal cells mostly express N-Cadherin (CDH2), while epithelial cells highly express E-Cadherin (CDH1). Among the non-classical cadherins, VE-Cadherin (CDH5) is expressed in endothelial cells and OB-Cadherin (CDH11) is expressed in osteoblasts. However, the expression level of cadherins may vary during different cellular processes, especially those that involve transition from one cellular state to another. For example, it is well established that the process of Epithelial to Mesenchymal Transition (EMT) is characterized by augmented expression of CDH2 and CDH11 and diminished expression of CDH1 [5–7]. Recent studies suggest that cadherin expression and cell-cell adhesion may also be critical in other transitions between cellular states such as lineage specification of stem cells or reprogramming of adult cells to a pluripotent state [8, 9].
Stem cell differentiation is affected by many soluble and insoluble signals in their local microenvironment. In addition to soluble growth factors, a number of elegant studies implicated cell-ExtraCellular Matrix (ECM) interactions and substrate mechanics in stem cell lineage commitment[10–17]. However, the mechanical and biochemical signals originating from cell-cell adhesion remain relatively unexplored in this context. Recent studies implicated adherens junctions in the maintenance of embryonic stem cell self-renewal potential, cellular reprogramming, hematopoietic stem cell engraftment and mesenchymal stem cell (MSC) differentiation into muscle [8, 9, 18]. Here we provide a brief review on the role of cadherins, in particular CDH2 and CDH11, in development and stem cell fate decisions. This is a relatively nascent field of stem cell biology that has the potential to guide the development of novel strategies to control stem cell fate decisions as well as to inspire biomimetic design of nanomaterials for tissue engineering and regeneration.
2. Adherens Junctions: signal transduction and mechanosensing
In general, classical cadherins including CDH2 and CDH11 have a common cytoplasmic domain and an ectodomain containing five tandem extracellular cadherin (EC) domains [19] (Fig. 1). The EC domains contain Ca2+ binding sites in which three Ca2+ ions work as inter-domain linkers, stabilizing the ectodomain structure and protecting it from proteolysis. [20–22] The outermost, EC1 domain regulates cadherin-cadherin interactions between adjacent cells, resulting in formation of adherens junctions between parallel opposing plasma membranes [23]. Specifically, CDH2 and CDH11 are mostly expressed in mesenchymal type cells such as fibroblasts and cardiac cells and mediate intercellular adhesion between cells of the same type e.g. myofibroblasts, or different cell types e.g. between myofibroblasts and cardiac cells[24].
Figure 1. Schematic representation of cadherin structure and downstream signaling.
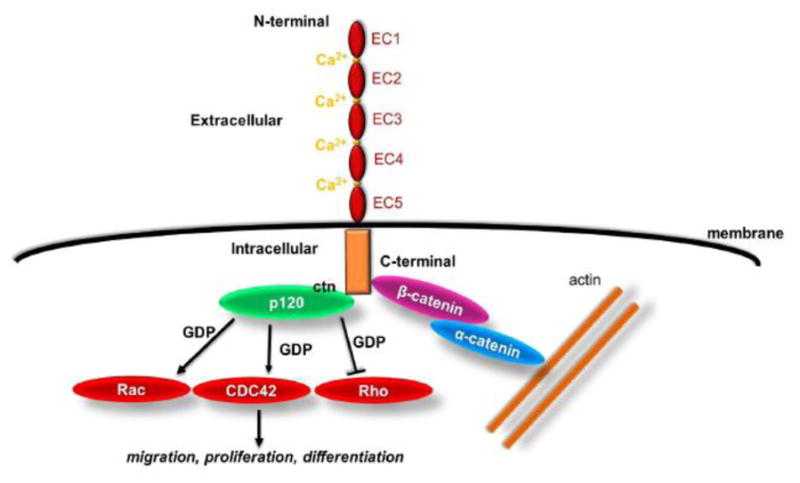
Cadherins contain five extra cellular (EC) domains linked by Ca2+ binding sites and one intracellular domain. Classical cadherin partners include to β-catenin, which binds to α-catenin linking the AJ complex to the actin cytoskeleton, as well as p120 catenin, which regulates small GTPases such as Rho, Rac, and Cdc42. Ultimately, cadherin engagement regulates many cellular processes including proliferation, migration and stem cell differentiation.
Interaction of cadherin-cadherin may lead to intercellular activation of cellular pathways, initiating through lamellipodial protrusions and is followed by the cadherin-catenin-actin cluster formation. The association of cadherin with catenin promotes and stabilizes the AJs, while actin polymerization leads to AJs expansion and maturation, further stabilizing and aligning adjacent cell membranes [4]. In particular, β-catenin binds to the cadherin cytoplasmic tail and interacts with α-catenin, which modulates the actin cytoskeleton. [1, 25] The intracellular domains of the cadherins also bind to p120 catenin, which links cadherin to microtubules[4] and regulates GTPases such as RhoA, Rac1 and Cdc42 [1, 26–31] (Fig. 1). Disrupting Rac or Rho activity perturbs AJ assembly, while Cdc42 affects AJ maintenance[32, 33]. The function of GTPases is linked to the cadherins and may control various cellular processes including polarization, migration and apoptosis. Specifically, CDH2 regulates spatially polarized signals through distinct p120 and β-catenin-dependent signaling pathways[34]. Interestingly, CDH2 mediated cell adhesion is important for collective 3D migration[35–37], while CDH11 is required for directional migration in vivo[38].
Several reports showed that cadherins are affected by growth factors and activate signaling pathways as a result of physical interactions with growth factor receptors. On exposure to shear stress, VE-cadherin binds to PECAM and vascular endothelial growth factor receptor (VEGFR2) and this complex may lead to integrin activation and actin cytoskeleton reorganization [39, 40]. Epidermal growth factor receptor (EGFR) forms a complex with CDH1, leading to activation of the mitogen-activated protein kinases (MAPK) pathway in epithelial cells [41, 42] with implications for cell survival [43] or EMT [44, 45]. Fibroblast growth factor receptors (FGFR) were shown to stimulate CDH2 during neurite outgrowth[46, 47], while FGF plays a critical role in the maintenance of vascular integrity by enhancing the stability of VE-cadherin at AJ sites [48]. Hepatocyte Growth Factor (HGF) modulates the expression of the cell adhesion molecule VE-cadherin and consequently endothelial cell motility, migration and angiogenesis[49]. Finally, TGF-β1 increases keratinocyte migration by increasing the levels of CDH2 and this action is counteracted by EGF [50].
Several reports have shown that cadherins are not only chemically but also mechanically regulated. Recently, our laboratory showed that substrate stiffness regulated AJ formation between epithelial cells in two-dimensional (2D) cultures and in three-dimensional (3D) epidermal tissues in vitro and in vivo by regulating the phosphorylation levels of the c-Janus N-terminal kinase (JNK) [51]. Rigid substrates led to JNK activation and AJ disassembly, while soft matrices suppressed JNK activity leading to AJ formation. The results held true in 3D bioengineered epidermis as well as in the epidermis of knockout (jnk1−/− or jnk2−/−) mice. In conclusion, we discovered that the JNK pathway mediated the effects of substrate stiffness on AJ formation in 2D and 3D context in vitro as well as in vivo. These findings shed light into the mechanisms of AJ formation and dissolution during tissue development and may provide novel guiding principles to control cell-cell vs. cell-substrate adhesion in 3D as a therapeutic strategy to promote tissue regeneration or inhibit tumor invasion.
Even though substrate stiffness and tethering is mostly known to affect focal adhesions [52–54], increasing evidence suggests that it may also affect cadherin-mediated intercellular adhesion [55, 56]. Substrate stiffness was implicated in cadherin-dependent collective cell migration through myosin-II contractility [57]. CDH2 is considered a mechanoresponsive adhesion receptor, as the forces transmitted through CDH2 junctions are comparable in magnitude to those sustained by integrin-ECM coupling[58]. In general, stiffer substrates lead to greater traction forces, larger cell-spread areas and better developed CDH2 junctions[56].
Finally, better understanding of cadherin based cell-cell interactions may be useful in development of scaffold-free tissue engineering strategies [59–61]. These strategies rely on directed cellular self-assembly using scaffold-free techniques including formation of spheroids or bioprinting, instead of biomaterial scaffolds to guide tissue formation, 3D organization and structure [62–66].
3. The role of CDH2 and CDH11 during development and morphogenesis
In the early stages of embryogenesis, the trophoblast giant cells are devoid of CDH2 or CDH11.[67] During gastrulation, the process generating the three germ cell layers, CDH11 is highly expressed enabling spatial recognition and segregation of cells as they move to generate primitive tissue structures[68–70]. At later stages as cells undergo EMT, CDH1 is downregulated, while CDH2 is upregulated and is important for proper left-right axis development [71]. In general, gastrulation gives rise to three germ layers: ectoderm, endoderm and mesoderm. CDH2 and CDH11 are absent in cells of the endodermal lineage [67] but play important roles in the development of ectodermal and mesodermal lineages as described below.
Ectodermal lineage
The ectoderm is the first germ layer to emerge during gastrulation. In vertebrates, the ectoderm is responsible for the formation of the nervous system and spinal cord. The nervous system is formed during neurulation, when the neural tube is transformed into a primitive structure and eventually into the central nervous system. Early in neural tube development, the notochord and the dorsal aorta do not express CDH11, which is expressed during the later stages of neural tube formation and is important for brain and spinal cord development[72, 73]. CDH11 is expressed in the limbic system of the brain, particularly in the hippocampus where it is thought to participate in the organization and stabilization of synaptic connections [74]. It is also expressed in the peripheral nervous system and, in particular, in motor and sensory axons during the period of active nerve elongation and path finding. [75, 76] CDH2 is present during neuroectoderm formation and is important for nervous system development. [77, 78] CDH2 knockout mice die on day 10 of gestation due to heart defects and malformed neural tubes, although tissue development appears normal up to this stage [79]. Others reported that CDH2 is involved in neuronal circuit maturation by contributing to axonal extension [5]. Finally, both CDH2 and CDH11 were shown to regulate neurite outgrowth through FGFR [80], PLCγ and CAM kinase pathways [81, 82].
Mesodermal lineage
Mesoderm is the middle developmental layer between the ectoderm and endoderm, which gives rise to skeleton, muscle, heart and bones. In early embryos, both CDH2 and CDH11 are found in the mesoderm [22, 83] albeit with different expression patterns. The head mesoderm expresses higher levels of CDH11 comparing to CDH2, while branchial arches express only CDH11.[5] CDH11 is present in all mesenchymal cells throughout the embryo such as mesenchymal cells of the stomach, intestine, pharynx, lung bud and shaft of ribs [67, 84–86] as well as mesenchymal stem cells originating from the pre-chondal and paraxial mesoderm and from neuroectodermal neural crest cells. CDH2 is also expressed in all mesenchymal and mesothelial tissues [87] and its expression is regulated by PDGF and FGF signaling [88].
4. The role of CDH2 and CDH11 in mesenchymal stem cell differentiation
Recently cadherins were found to regulate stem cell maintenance and differentiation. CDH1 was necessary for maintaining pluripotency of embryonic stem cells as well as for cellular reprogramming, where ectopic expression of CDH1 could substitute for the pluripotency factor Oct4 [8]. Interestingly, CDH2 was implicated in long-term engraftment of hematopoietic stem cells and establishment of hematopoiesis after bone marrow transplantation [18] but its exact role remains controversial. Some studies suggested that it might be necessary as inhibition of cadherin-mediated homophilic and heterophilic adhesion reduced the long-term repopulation activity of Hematopoietic Stem Cells (HSCs) [89]. However, others reported that CDH2 conditional knockout mice do not show defects in HSC number or function [90].
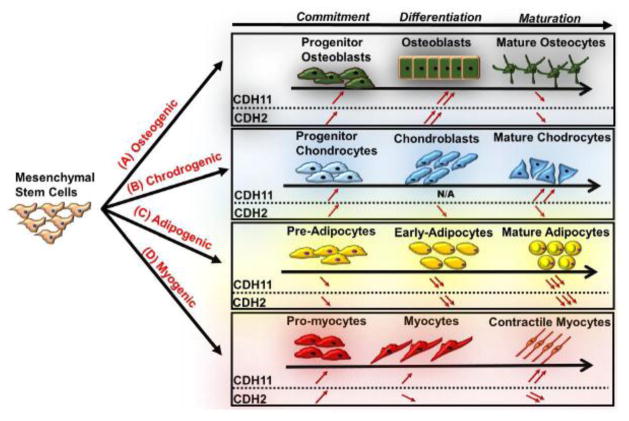
On the other hand, accumulating evidence suggests that both cadherins play important roles in MSC differentiation. MSC provide an excellent cell source for cellular therapies to treat bone and cartilage disorders [91, 92], myocardial infarction, stroke [93, 94], rheumatoid arthritis [95], acute lung injury [96, 97], graft-versus-host disease [98] and skin-graft rejection [99] among others. The use of MSC for tissue repair requires the migration and homing to the site of damaged tissue and it has been shown that both the migratory and proliferation potential of these cells are affected by CDH2 and CDH11 [100, 101]. MSCs have also been shown not only to have differentiation potential but also potent anti-inflammatory effects [102], which are enhanced when cultured as 3D spheroid aggregates [103, 104]. Interestingly, both CDH2 and CDH11 were shown to be critical in the response of synovial fibroblasts to inflammation [105, 106], suggesting that cadherins may also be important in mediating the anti-inflammatory effects of MSC. Finally, CDH2 and CDH11 have been shown to be critical for MSC differentiation and their expression levels are regulated differently in osteogenic, chondrogenic or myogenic lineages as described below (Fig. 2).
Figure 2. Schematic representation of CDH2 and CDH11 expression during MSC lineage commitment.
CDH2 and CDH11 expression levels during MSC commitment, differentiation and maturation towards (A) Osteogenic; (B) Chondrogenic; (C) Adipogenic; or (D) Myogenic Lineages. Upward or downward pointing arrows indicate increased or decreased expression, respectively.
I. Osteogenic Lineage
CDH2 and CDH11 are highly expressed during MSC osteogenic differentiation [107] and several pro-osteogenic factors are known to affect their expression. For example, well-known osteogenic inducers, such as BMP-2, parathyroid hormone (PTH), bFGF and phorbol ester increased the levels of these cadherins [108–110]. On the other hand, Vitamin D decreased expression of CDH2 [111] and dexamethasone inhibited the expression of both CDH2 and CDH11 mRNA in human osteoprogenitor marrow stromal cells (BMC) [112]. Interestingly, both CDH2 and CDH11 were downregulated in mature osteocytes [113].
Loss-of-function studies provided definitive data supporting the role of both cadherins in bone formation. Blocking of CDH2 or CDH11 with inhibitory peptides prevented osteoblastic differentiation in vitro [108, 113, 114]. In agreement, CDH11 knockout null mice showed modest osteopenia by three months of age as signified by decreased mineralizing surface and trabecular bone volume [115]. The role of each cadherin in osteogenesis was further dissected by using double knockout mice (Chd2+/−;Cdh11−/−) and showed that although both CDH2 and CDH11 are important for osteogenesis, their contributions were mediated by distinct mechanisms. Specifically, CDH11 was pro-osteogenic but dispensable for postnatal skeletal growth; on the other hand, CDH2 was necessary for maintaining the precursor osteoblast pool [116]. This result might explain why overexpression of CDH2 promoted migration but inhibited osteogenesis as evidenced by decreased expression of osteogenic genes osteopontin, osteocalcin, RunX2, alkaline phosphatase (ALP) and BMP-2, as well as ALP activity and calcium deposition in BM-MSC [100].
II. Chondrogenic Lineage
During chondrogenesis CDH2 and Sox9 were upregulated by the action of paracrine factors like TGF-β, FGFs, or BMPs, and the transcription factor Sox9 further increased the CDH2 promoter activity [117]. CDH2 mediated cell-cell interactions and increased MSC aggregation, which in turn promoted differentiation into the chondrogenic lineage [118, 119]. CDH2 was required for the initial condensation phase but decreased significantly during terminal chondrogenic differentiation. [120, 121] In agreement, it has been reported that the cleavage of CDH2 was required during chondrogenic differentiation [122], while inhibition of commitment to chondrogenic lineage by the Wnt7a inhibitor led to enhanced CDH2 expression and stabilization of AJs. [123–125] Interestingly, loss of CDH2 led to increased levels of CDH11, suggesting that compensatory mechanisms might be at work [126].
III. Adipogenic Lineage
During adipogenesis, CDH2 and CDH11 were downregulated and mature adipocytes did not express either of cadherin [127, 128]. In addition, CDH11 knockdown induced adipogenic gene expression (e.g. PPARγ) and differentiation, suggesting that CDH11 might inhibit adipogenesis.
IV. Myogenic Lineage
CDH2 and CDH11 are also important during myogenic differentiation. High cell density was shown to promote myoblast differentiation, suggesting that cadherin mediated cell–cell contact might affect myogenesis [129, 130]. CDH2 and CDH11 also play important role in wound healing when fibroblasts turn into myofibroblasts to increase wound contraction and promote wound closure [84, 131–134]. Interestingly, CDH11 was upregulated in vascular smooth muscle cells (SMCs) in response to injury, while its inhibition reduced SMC proliferation and migration [85].
Recently, our group reported that CDH11 but not CDH2 was necessary for MSC differentiation into SMCs [9] (Fig. 3). CDH11 engagement regulated MSC to SMC differentiation via two pathways. One pathway was dependent on TGFβ receptor II (TGFβRII) but independent of SMAD2/3. The second pathway involved activation of Rho-associated protein kinase (ROCK), which in turn induced expression of serum response factor (SRF) and SMC proteins such as alpha smooth muscle actin (αSMA), calponin and myosin heavy chain (MYH11). Increased expression of SRF resulted in increased expression of CDH11, indicating the presence of a positive feedback loop that led to increased CDH11 engagement and subsequent commitment of MSC to the SMC fate (Fig. 3). Experiments with CDH11-null (Cdh11−/−) mice verified the role of CDH11 in SMC function as vascular and urogenital tissues of these animals exhibited significantly reduced levels of SMC proteins and most notably, diminished contractility as compared to wild-type controls. These findings are novel and surprising as Cdh11−/− mice develop normally, are fertile and display no obvious phenotype other than modest osteopenia[115, 127, 128] and decreased pulmonary fibrosis after lung injury[135]. More work is required to understand the mechanism through which CDH11 affects SMC function and the potential implications of CDH11 loss in cardiovascular, urogenital, gastrointestinal and other SMC containing tissues.
Figure 3. CDH11 mediated AJ formation promotes MSC differentiation into SMC [9].
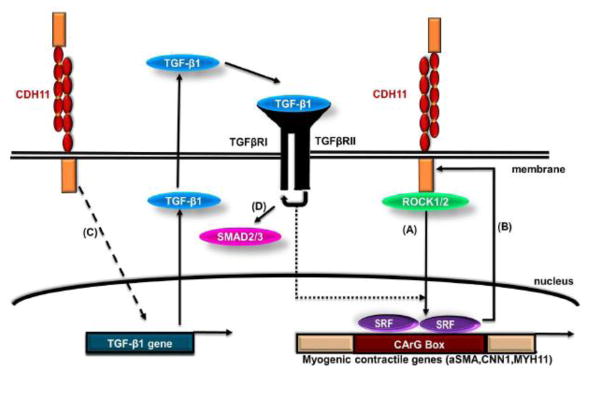
(A) Engagement of CDH11 activates the ROCK pathway, which in turn activates SRF leading to increased expression of SMC genes. (B) SRF controls the level of CDH11 expression through a positive feedback loop further promoting intercellular adhesion. (C) CDH11 engagement also increases TGF-β1 expression further promoting SMC differentiation (D) through a Smad2/3 independent pathway.
5. Engineering cell-cell adhesion to direct stem cell fate decisions
The findings that we described above show that CDH2 and CDH11 play important roles in stem cell lineage specification, and therefore, could be used to develop technologies to control stem cell differentiation by exploiting cell-cell interactions. To this end, we propose the following strategies (Table 1) to capitalize on the effects of cadherin-mediated intercellular adhesion: (i) Engineering cadherin surfaces to control stem cell differentiation; (ii) Engineering surface microtopology to control the extent of cell-cell adhesion and signaling.
Table 1.
Engineering cell adhesion strategies to direct stem cell fate decision
| Strategy | Approach | MSC Differentiation | Advantages | Ref. |
|---|---|---|---|---|
| Engineering cadherin surfaces | Cadherin immobilization to surfaces |
CDH2-CDH2 interactions: Increased osteo-, chondro- and myogenic differentiation Decreased adipogenic differentiation |
|
[134–140, 151] |
|
CDH11-CDH11 interactions: Increased myogenic and osteogenic differentiation | ||||
| Engineering surface microtopology | Microfabrication/Micropatterning |
Large micro-island:
Upregulate chondrogenic and myogenic differentiation Small micro-island: Increase adipogenic fate |
|
[137–146] [12–14] |
(i) Engineering cadherin surfaces to control stem cell differentiation
It has been shown that immobilized cadherins induced similar signaling cascades in epithelial cells as CDH1 engagement during cell-cell contact. Cadherin immobilization was facilitated by generating fusion proteins between cadherins with the Fc antibody fragment that enables protein immobilization to the surface. In addition, to generating functional surfaces, immobilized cadherins can be used to distinguish cadherin-mediated signaling pathways from pathways activated by the engagement of other junctional proteins e.g. connexins, which usually follows AJ formation during cell-cell contact [136].
This approach has been used to immobilize several cadherins including CDH1, CDH2 and CDH11 to regulate cellular behavior. Specifically, CDH1-Fc activated Rac1 and decreased RhoA activity in epithelial cells [136–138] and improved hepatocyte DNA synthesis and proliferation [139, 140]. Similarly, immobilization of CDH2-Fc retained the adhesive properties of native CDH2, resulting in recruitment of β-catenin, α-catenin and p120 at the cell-cell contact sites [141]. CDH2-Fc coated beads triggered myoblast maturation as evidenced by increased expression of myogenic regulators such as SRF [142, 143]. Interestingly, CDH2 in lipid bilayer membranes induced mesenchymal condensation of osteochondrogenic progenitors and suppressed adipogenic differentiation. [144] Likewise, CDH11-Fc proteins formed dimers that were shown to be functional i.e. engaged in strong homotypic CDH11 interactions [145] and promoted binding of CDH11-expressing L cells. [145, 146] Also, culture of MSCs on surface immobilized fusion protein between a fibronectin domain (rFN) and CDH11 (rFN/CDH11) significantly enhanced osteogenic differentiation.[147] Finally, preliminary experiments in our laboratory showed that immobilized cadherins promoted MSC differentiation into SMC cells in a dose dependent manner, thereby providing control of differentiation by surface presentation and density. Collectively, these studies suggest the cadherin immobilization can be employed to direct and/or fine tune stem cell fate decisions and therefore, can be a useful strategy enabling functionalization of biomaterial scaffolds for tissue engineering and regenerative medicine.
(ii) Engineering surface microtopology to control the extent of cell-cell adhesion and signaling
Microfabrication technology offers the possibility to control the extent cell-cell adhesion at the micro- or nanometer scale. This approach has been used extensively to control cell-matrix interactions, which have been shown to be critical in stem cell differentiation [12, 13, 148–151]. Fewer studies have used geometric micropatterning to control the extent of cell-cell adhesion and evaluate its effects on stem cell differentiation [152].
It was shown that the size of micro-islands correlated with the level of cell spreading and CDH2 expression leading to MSC differentiation into the myogenic or chondrogenic lineages on the larger islands but adipogenic lineage on the small ones.[14] Similarly, by controlling the geometry and size of micro-islands it was shown that increased cell contact increased the extent of osteogenic differentiation [153, 154]. However, attempts to control cell-cell interactions by varying the size of micropatterns are compounded by the fact that cell density and therefore the degree of cell spreading change with island size, making it difficult to separate the effects of cell-cell vs. cell-substrate adhesion. Interestingly, novel geometries have been employed to control the extent of cell-cell contact independent of cell density or the cell spreading area [155–157], and therefore, may be used to determine the relationship between the extent of intercellular adhesion and stem cell fate commitment.
6. Monitoring intercellular adhesion mediated stem cell lineage specification in real time
Understanding how intercellular adhesion affects stem cell fate decisions requires methods to interrogate stem cell differentiation in real time and in a quantitative manner. In particular, methods to monitor individual cells may be particularly useful in experiments that involve small numbers of cells on micropatterned surfaces, thereby making traditional assays such as Western Blot and PCR challenging. In addition, monitoring single cells is useful in addressing issues of heterogeneity in embryonic, induced pluripotent or adult stem cells populations and therefore, in distinguishing between cells with varying differentiation potential.
To this end, our laboratory developed LentiViral Arrays (LVA) to monitor gene or pathway activation during stem cell differentiation. We designed a novel lentiviral dual promoter vector (LVDP) vector that enables quantitative measurements of the activity of a gene promoter (Pr) or a transcription factor (TF) binding site (Response Element, RE) independent of the number of gene copies per cell [158]. We also designed a second lentiviral vector (shLVDP) that enables dynamic monitoring of Pr/RE activity with concomitant gene knockdown in a doxycycline (Dox)-regulatable manner, thereby enabling discovery of genes that may be involved in stem cell differentiation.[159] In addition, the envelope of lentiviral particles was engineered to bind covalently to fibrin hydrogels during polymerization [160, 161], thereby enabling generation of lentiviral arrays (LVA) that were employed to measure the activity of several Pr/RE participating in the inflammatory response[162]. More recently, we generated a library of Pr/RE to monitor MSC differentiation towards adipogenic, osteogenic, chondrogenic or myogenic lineages and used it to identify novel pathways that may be involved in lineage specification[163, 164]. Potentially, this technology may be combined with novel microfabrication methods to determine how the extent of intercellular adhesion influences stem cell specification decisions of adult stem cells, cancer stem cells or hiPSC and potentially also the pluripotency networks that are critical for cellular reprogramming.
7. Conclusion and future perspectives
Although many studies have focused on the effects of substrate stiffness on stem cell biology, the role of intercellular adhesion forces in guiding stem cell self-renewal or differentiation has been relatively unexplored. In this review, we focused on CDH2 and CDH11 as regulators of stem cell fate decisions. Although evidence that cadherins are important has been surfacing, more work is necessary to understand how intercellular adhesion affects MSC differentiation and reveal some of the molecular pathways guiding this process. These studies may also provide design parameters for guiding MSC fate by controlling the extent of cadherin-mediated adhesion with implications for tissue engineering and regenerative medicine.
Highlights.
The role of CDH11 and CDH2 in development and morphogenesis.
The role of CDH2 and CDH11 during MSC lineage commitment.
CDH11 mediated AJ formation promotes MSC differentiation into SMC.
Directing stem cell fate by controlling intracellular adhesion.
Engineering cell-cell adhesion to direct stem cell fate decisions.
Acknowledgments
This work was supported in part by a grant from the National Science Foundation (CBET-1403086) to S.T.A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cavallaro U, Dejana E. Adhesion molecule signalling: not always a sticky business. Nat Rev Mol Cell Biol. 2011;12:189–197. doi: 10.1038/nrm3068. [DOI] [PubMed] [Google Scholar]
- 2.Niessen CM, Gumbiner BM. Cadherin-mediated cell sorting not determined by binding or adhesion specificity. J Cell Biol. 2002;156:389–399. doi: 10.1083/jcb.200108040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Bennett SA, Wang L. Role of E-cadherin and other cell adhesion molecules in survival and differentiation of human pluripotent stem cells. Cell adhesion & migration. 2012;6:59–70. doi: 10.4161/cam.19583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11:502–514. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- 5.Kimura Y, Matsunami H, Inoue T, Shimamura K, Uchida N, Ueno T, Miyazaki T, Takeichi M. Cadherin-11 expressed in association with mesenchymal morphogenesis in the head, somite, and limb bud of early mouse embryos. Dev Biol. 1995;169:347–358. doi: 10.1006/dbio.1995.1149. [DOI] [PubMed] [Google Scholar]
- 6.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomita K, van Bokhoven A, van Leenders GJ, Ruijter ET, Jansen CF, Bussemakers MJ, Schalken JA. Cadherin switching in human prostate cancer progression. Cancer Res. 2000;60:3650–3654. [PubMed] [Google Scholar]
- 8.Redmer T, Diecke S, Grigoryan T, Quiroga-Negreira A, Birchmeier W, Besser D. E-cadherin is crucial for embryonic stem cell pluripotency and can replace OCT4 during somatic cell reprogramming. EMBO Rep. 2011;12:720–726. doi: 10.1038/embor.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alimperti S, You H, George T, Agarwal SK, Andreadis ST. Cadherin-11 regulates both mesenchymal stem cell differentiation into smooth muscle cells and the development of contractile function in vivo. Journal of cell science. 2014;127:2627–2638. doi: 10.1242/jcs.134833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buxboim A, Discher DE. Stem cells feel the difference. Nat Methods. 2010;7:695–697. doi: 10.1038/nmeth0910-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buxboim A, Ivanovska IL, Discher DE. Matrix elasticity, cytoskeletal forces and physics of the nucleus: how deeply do cells ‘feel’ outside and in? J Cell Sci. 2010;123:297–308. doi: 10.1242/jcs.041186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 13.Engler AJ, Sweeney HL, Discher DE, Schwarzbauer JE. Extracellular matrix elasticity directs stem cell differentiation. Journal of musculoskeletal & neuronal interactions. 2007;7:335. [PubMed] [Google Scholar]
- 14.Gao L, McBeath R, Chen CS. Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-cadherin. Stem Cells. 2010;28:564–572. doi: 10.1002/stem.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 16.Treiser MD, Yang EH, Gordonov S, Cohen DM, Androulakis IP, Kohn J, Chen CS, Moghe PV. Cytoskeleton-based forecasting of stem cell lineage fates. Proc Natl Acad Sci U S A. 2010;107:610–615. doi: 10.1073/pnas.0909597107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hosokawa K, Arai F, Yoshihara H, Iwasaki H, Nakamura Y, Gomei Y, Suda T. Knockdown of N-cadherin suppresses the long-term engraftment of hematopoietic stem cells. Blood. 2010;116:554–563. doi: 10.1182/blood-2009-05-224857. [DOI] [PubMed] [Google Scholar]
- 19.Brasch J, Harrison OJ, Honig B, Shapiro L. Thinking outside the cell: how cadherins drive adhesion. Trends Cell Biol. 2012;22:299–310. doi: 10.1016/j.tcb.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boggon TJ, Murray J, Chappuis-Flament S, Wong E, Gumbiner BM, Shapiro L. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science. 2002;296:1308–1313. doi: 10.1126/science.1071559. [DOI] [PubMed] [Google Scholar]
- 21.Pokutta S, Herrenknecht K, Kemler R, Engel J. Conformational changes of the recombinant extracellular domain of E-cadherin upon calcium binding. Eur J Biochem. 1994;223:1019–1026. doi: 10.1111/j.1432-1033.1994.tb19080.x. [DOI] [PubMed] [Google Scholar]
- 22.Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro L, Weis WI. Structure and biochemistry of cadherins and catenins. Cold Spring Harb Perspect Biol. 2009;1:a003053. doi: 10.1101/cshperspect.a003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson SA, Blazeski A, Copeland CR, Cohen DM, Chen CS, Reich DM, Tung L. Acute slowing of cardiac conduction in response to myofibroblast coupling to cardiomyocytes through N-cadherin. J Mol Cell Cardiol. 2014;68:29–37. doi: 10.1016/j.yjmcc.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stepniak E, Radice GL, Vasioukhin V. Adhesive and signaling functions of cadherins and catenins in vertebrate development. Cold Spring Harb Perspect Biol. 2009;1:a002949. doi: 10.1101/cshperspect.a002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reynolds AB, Carnahan RH. Regulation of cadherin stability and turnover by p120ctn: implications in disease and cancer. Semin Cell Dev Biol. 2004;15:657–663. doi: 10.1016/j.semcdb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Yonemura S. Cadherin-actin interactions at adherens junctions. Curr Opin Cell Biol. 2011;23:515–522. doi: 10.1016/j.ceb.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Grosheva I, Shtutman M, Elbaum M, Bershadsky AD. p120 catenin affects cell motility via modulation of activity of Rho-family GTPases: a link between cell-cell contact formation and regulation of cell locomotion. J Cell Sci. 2001;114:695–707. doi: 10.1242/jcs.114.4.695. [DOI] [PubMed] [Google Scholar]
- 29.Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, Zheng Y, Reynolds AB. Inhibition of RhoA by p120 catenin. Nat Cell Biol. 2000;2:637–644. doi: 10.1038/35023588. [DOI] [PubMed] [Google Scholar]
- 30.Anastasiadis PZ, Reynolds AB. The p120 catenin family: complex roles in adhesion, signaling and cancer. J Cell Sci. 2000;113(Pt 8):1319–1334. doi: 10.1242/jcs.113.8.1319. [DOI] [PubMed] [Google Scholar]
- 31.Noren NK, Liu BP, Burridge K, Kreft B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol. 2000;150:567–580. doi: 10.1083/jcb.150.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yap AS, Kovacs EM. Direct cadherin-activated cell signaling: a view from the plasma membrane. J Cell Biol. 2003;160:11–16. doi: 10.1083/jcb.200208156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braga VM. Cell-cell adhesion and signalling. Curr Opin Cell Biol. 2002;14:546–556. doi: 10.1016/s0955-0674(02)00373-3. [DOI] [PubMed] [Google Scholar]
- 34.Ouyang M, Lu S, Kim T, Chen CE, Seong J, Leckband DE, Wang F, Reynolds AB, Schwartz MA, Wang Y. N-cadherin regulates spatially polarized signals through distinct p120ctn and beta-catenin-dependent signalling pathways. Nature communications. 2013;4:1589. doi: 10.1038/ncomms2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peglion F, Llense F, Etienne-Manneville S. Adherens junction treadmilling during collective migration. Nat Cell Biol. 2014;16:639–651. doi: 10.1038/ncb2985. [DOI] [PubMed] [Google Scholar]
- 36.Shih W, Yamada S. N-cadherin as a key regulator of collective cell migration in a 3D environment. Cell adhesion & migration. 2012;6:513–517. doi: 10.4161/cam.21766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shih W, Yamada S. N-cadherin-mediated cell-cell adhesion promotes cell migration in a three-dimensional matrix. J Cell Sci. 2012;125:3661–3670. doi: 10.1242/jcs.103861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Becker SF, Mayor R, Kashef J. Cadherin-11 mediates contact inhibition of locomotion during Xenopus neural crest cell migration. PLoS One. 2013;8:e85717. doi: 10.1371/journal.pone.0085717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shay-Salit A, Shushy M, Wolfovitz E, Yahav H, Breviario F, Dejana E, Resnick N. VEGF receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proc Natl Acad Sci U S A. 2002;99:9462–9467. doi: 10.1073/pnas.142224299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 41.Pece S, Gutkind JS. Signaling from E-cadherins to the MAPK pathway by the recruitment and activation of epidermal growth factor receptors upon cell-cell contact formation. J Biol Chem. 2000;275:41227–41233. doi: 10.1074/jbc.M006578200. [DOI] [PubMed] [Google Scholar]
- 42.Hoschuetzky H, Aberle H, Kemler R. Beta-catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J Cell Biol. 1994;127:1375–1380. doi: 10.1083/jcb.127.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen X, Kramer RH. Adhesion-mediated squamous cell carcinoma survival through ligand-independent activation of epidermal growth factor receptor. Am J Pathol. 2004;165:1315–1329. doi: 10.1016/S0002-9440(10)63390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wendt MK, Smith JA, Schiemann WP. Transforming growth factor-beta-induced epithelial-mesenchymal transition facilitates epidermal growth factor-dependent breast cancer progression. Oncogene. 2010;29:6485–6498. doi: 10.1038/onc.2010.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jia J, Zhang W, Liu JY, Chen G, Liu H, Zhong HY, Liu B, Cai Y, Zhang JL, Zhao YF. Epithelial mesenchymal transition is required for acquisition of anoikis resistance and metastatic potential in adenoid cystic carcinoma. PLoS One. 2012;7:e51549. doi: 10.1371/journal.pone.0051549. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Williams EJ, Furness J, Walsh FS, Doherty P. Activation of the FGF receptor underlies neurite outgrowth stimulated by L1, N-CAM, and N-cadherin. Neuron. 1994;13:583–594. doi: 10.1016/0896-6273(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 47.Williams G, Williams EJ, Doherty P. Dimeric versions of two short N-cadherin binding motifs (HAVDI and INPISG) function as N-cadherin agonists. J Biol Chem. 2002;277:4361–4367. doi: 10.1074/jbc.M109185200. [DOI] [PubMed] [Google Scholar]
- 48.Hatanaka K, Lanahan AA, Murakami M, Simons M. Fibroblast growth factor signaling potentiates VE-cadherin stability at adherens junctions by regulating SHP2. PLoS One. 2012;7:e37600. doi: 10.1371/journal.pone.0037600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin TA, Mansel R, Jiang WG. Hepatocyte growth factor modulates vascular endothelial-cadherin expression in human endothelial cells. Clin Cancer Res. 2001;7:734–737. [PubMed] [Google Scholar]
- 50.Diamond ME, Sun L, Ottaviano AJ, Joseph MJ, Munshi HG. Differential growth factor regulation of N-cadherin expression and motility in normal and malignant oral epithelium. J Cell Sci. 2008;121:2197–2207. doi: 10.1242/jcs.021782. [DOI] [PubMed] [Google Scholar]
- 51.You H, Padmashali RM, Ranganathan A, Lei P, Girnius N, Davis RJ, Andreadis ST. JNK regulates compliance-induced adherens junctions formation in epithelial cells and tissues. J Cell Sci. 2013;126:2718–2729. doi: 10.1242/jcs.122903. [DOI] [PubMed] [Google Scholar]
- 52.Trappmann B, Gautrot JE, Connelly JT, Strange DG, Li Y, Oyen ML, Cohen Stuart MA, Boehm H, Li B, Vogel V, Spatz JP, Watt FM, Huck WT. Extracellular-matrix tethering regulates stem-cell fate. Nature materials. 2012;11:642–649. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 53.Wen JH, Vincent LG, Fuhrmann A, Choi YS, Hribar KC, Taylor-Weiner H, Chen S, Engler AJ. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nature materials. 2014;13:979–987. doi: 10.1038/nmat4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smutny M, Yap AS. Neighborly relations: cadherins and mechanotransduction. J Cell Biol. 2010;189:1075–1077. doi: 10.1083/jcb.201005151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ladoux B, Anon E, Lambert M, Rabodzey A, Hersen P, Buguin A, Silberzan P, Mege RM. Strength dependence of cadherin-mediated adhesions. Biophys J. 2010;98:534–542. doi: 10.1016/j.bpj.2009.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ng MR, Besser A, Danuser G, Brugge JS. Substrate stiffness regulates cadherin-dependent collective migration through myosin-II contractility. J Cell Biol. 2012;199:545–563. doi: 10.1083/jcb.201207148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chopra A, Tabdanov E, Patel H, Janmey PA, Kresh JY. Cardiac myocyte remodeling mediated by N-cadherin-dependent mechanosensing. Am J Physiol Heart Circ Physiol. 2011;300:H1252–1266. doi: 10.1152/ajpheart.00515.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dvir-Ginzberg M, Gamlieli-Bonshtein I, Agbaria R, Cohen S. Liver tissue engineering within alginate scaffolds: effects of cell-seeding density on hepatocyte viability, morphology, and function. Tissue Eng. 2003;9:757–766. doi: 10.1089/107632703768247430. [DOI] [PubMed] [Google Scholar]
- 60.Mertsching H, Walles T, Hofmann M, Schanz J, Knapp WH. Engineering of a vascularized scaffold for artificial tissue and organ generation. Biomaterials. 2005;26:6610–6617. doi: 10.1016/j.biomaterials.2005.04.048. [DOI] [PubMed] [Google Scholar]
- 61.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nature materials. 2009;8:457–470. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 62.Schiele NR, Koppes RA, Chrisey DB, Corr DT. Engineering cellular fibers for musculoskeletal soft tissues using directed self-assembly. Tissue Eng Part A. 2013;19:1223–1232. doi: 10.1089/ten.tea.2012.0321. [DOI] [PubMed] [Google Scholar]
- 63.Baraniak PR, McDevitt TC. Scaffold-free culture of mesenchymal stem cell spheroids in suspension preserves multilineage potential. Cell Tissue Res. 2012;347:701–711. doi: 10.1007/s00441-011-1215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Norotte C, Marga FS, Niklason LE, Forgacs G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 2009;30:5910–5917. doi: 10.1016/j.biomaterials.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stevens KR, Kreutziger KL, Dupras SK, Korte FS, Regnier M, Muskheli V, Nourse MB, Bendixen K, Reinecke H, Murry CE. Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc Natl Acad Sci U S A. 2009;106:16568–16573. doi: 10.1073/pnas.0908381106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Napolitano AP, Dean DM, Man AJ, Youssef J, Ho DN, Rago AP, Lech MP, Morgan JR. Scaffold-free three-dimensional cell culture utilizing micromolded nonadhesive hydrogels. Biotechniques. 2007;43:494, 496–500. doi: 10.2144/000112591. [DOI] [PubMed] [Google Scholar]
- 67.Simonneau L, Kitagawa M, Suzuki S, Thiery JP. Cadherin 11 expression marks the mesenchymal phenotype: towards new functions for cadherins? Cell Adhes Commun. 1995;3:115–130. doi: 10.3109/15419069509081281. [DOI] [PubMed] [Google Scholar]
- 68.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 69.Guillot C, Lecuit T. Mechanics of epithelial tissue homeostasis and morphogenesis. Science. 2013;340:1185–1189. doi: 10.1126/science.1235249. [DOI] [PubMed] [Google Scholar]
- 70.Rossant J, Tam PP. Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development. 2009;136:701–713. doi: 10.1242/dev.017178. [DOI] [PubMed] [Google Scholar]
- 71.Garcia-Castro MI, Vielmetter E, Bronner-Fraser M. N-Cadherin a cell adhesion molecule involved in establishment of embryonic left-right asymmetry. Science. 2000;288:1047–1051. doi: 10.1126/science.288.5468.1047. [DOI] [PubMed] [Google Scholar]
- 72.Suzuki SC, Inoue T, Kimura Y, Tanaka T, Takeichi M. Neuronal circuits are subdivided by differential expression of type-II classic cadherins in postnatal mouse brains. Mol Cell Neurosci. 1997;9:433–447. doi: 10.1006/mcne.1997.0626. [DOI] [PubMed] [Google Scholar]
- 73.Marthiens V, Gavard J, Lambert M, Mege RM. Cadherin-based cell adhesion in neuromuscular development. Biol Cell. 2002;94:315–326. doi: 10.1016/s0248-4900(02)00005-9. [DOI] [PubMed] [Google Scholar]
- 74.Manabe T, Togashi H, Uchida N, Suzuki SC, Hayakawa Y, Yamamoto M, Yoda H, Miyakawa T, Takeichi M, Chisaka O. Loss of cadherin-11 adhesion receptor enhances plastic changes in hippocampal synapses and modifies behavioral responses. Mol Cell Neurosci. 2000;15:534–546. doi: 10.1006/mcne.2000.0849. [DOI] [PubMed] [Google Scholar]
- 75.Marthiens V, Padilla F, Lambert M, Mege RM. Complementary expression and regulation of cadherins 6 and 11 during specific steps of motoneuron differentiation. Mol Cell Neurosci. 2002;20:458–475. doi: 10.1006/mcne.2002.1130. [DOI] [PubMed] [Google Scholar]
- 76.Padilla F, Broders F, Nicolet M, Mege RM. Cadherins M, 11, and 6 expression patterns suggest complementary roles in mouse neuromuscular axis development. Mol Cell Neurosci. 1998;11:217–233. doi: 10.1006/mcne.1998.0681. [DOI] [PubMed] [Google Scholar]
- 77.Kadowaki M, Nakamura S, Machon O, Krauss S, Radice GL, Takeichi M. N-cadherin mediates cortical organization in the mouse brain. Dev Biol. 2007;304:22–33. doi: 10.1016/j.ydbio.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 78.Redies C. Cadherins in the central nervous system. Prog Neurobiol. 2000;61:611–648. doi: 10.1016/s0301-0082(99)00070-2. [DOI] [PubMed] [Google Scholar]
- 79.Radice GL, Rayburn H, Matsunami H, Knudsen KA, Takeichi M, Hynes RO. Developmental defects in mouse embryos lacking N-cadherin. Dev Biol. 1997;181:64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- 80.Boscher C, Mege RM. Cadherin-11 interacts with the FGF receptor and induces neurite outgrowth through associated downstream signalling. Cell Signal. 2008;20:1061–1072. doi: 10.1016/j.cellsig.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 81.Bixby JL, Grunwald GB, Bookman RJ. Ca2+ influx and neurite growth in response to purified N-cadherin and laminin. J Cell Biol. 1994;127:1461–1475. doi: 10.1083/jcb.127.5.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Riehl R, Johnson K, Bradley R, Grunwald GB, Cornel E, Lilienbaum A, Holt CE. Cadherin function is required for axon outgrowth in retinal ganglion cells in vivo. Neuron. 1996;17:837–848. doi: 10.1016/s0896-6273(00)80216-0. [DOI] [PubMed] [Google Scholar]
- 83.Hatta K, Takeichi M. Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature. 1986;320:447–449. doi: 10.1038/320447a0. [DOI] [PubMed] [Google Scholar]
- 84.Kuijpers KA, Heesakkers JP, Jansen CF, Schalken JA. Cadherin-11 is expressed in detrusor smooth muscle cells and myofibroblasts of normal human bladder. Eur Urol. 2007;52:1213–1221. doi: 10.1016/j.eururo.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 85.Monahan TS, Andersen ND, Panossian H, Kalish JA, Daniel S, Shrikhande GV, Ferran C, Logerfo FW. A novel function for cadherin 11/osteoblast-cadherin in vascular smooth muscle cells: modulation of cell migration and proliferation. J Vasc Surg. 2007;45:581–589. doi: 10.1016/j.jvs.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 86.Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- 87.Derycke LD, Bracke ME. N-cadherin in the spotlight of cell-cell adhesion, differentiation, embryogenesis, invasion and signalling. Int J Dev Biol. 2004;48:463–476. doi: 10.1387/ijdb.041793ld. [DOI] [PubMed] [Google Scholar]
- 88.Yang X, Chrisman H, Weijer CJ. PDGF signalling controls the migration of mesoderm cells during chick gastrulation by regulating N-cadherin expression. Development. 2008;135:3521–3530. doi: 10.1242/dev.023416. [DOI] [PubMed] [Google Scholar]
- 89.Hosokawa K, Arai F, Yoshihara H, Iwasaki H, Hembree M, Yin T, Nakamura Y, Gomei Y, Takubo K, Shiama H, Matsuoka S, Li L, Suda T. Cadherin-based adhesion is a potential target for niche manipulation to protect hematopoietic stem cells in adult bone marrow. Cell Stem Cell. 2010;6:194–198. doi: 10.1016/j.stem.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 90.Kiel MJ, Acar M, Radice GL, Morrison SJ. Hematopoietic stem cells do not depend on N-cadherin to regulate their maintenance. Cell Stem Cell. 2009;4:170–179. doi: 10.1016/j.stem.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10:199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 93.Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, Gao Q, Shen LH, Zhang J, Lu M, Chopp M. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- 94.Wang L, Li Y, Chen X, Chen J, Gautam SC, Xu Y, Chopp M. MCP-1, MIP-1, IL-8 and ischemic cerebral tissue enhance human bone marrow stromal cell migration in interface culture. Hematology. 2002;7:113–117. doi: 10.1080/10245330290028588. [DOI] [PubMed] [Google Scholar]
- 95.Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56:1175–1186. doi: 10.1002/art.22511. [DOI] [PubMed] [Google Scholar]
- 96.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 97.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 2007;104:11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dander E, Lucchini G, Vinci P, Introna M, Masciocchi F, Perseghin P, Balduzzi A, Bonanomi S, Longoni D, Gaipa G, Belotti D, Parma M, Algarotti A, Capelli C, Golay J, Rovelli A, Rambaldi A, Biondi A, Biagi E, D’Amico G. Mesenchymal stromal cells for the treatment of graft-versus-host disease: understanding the in vivo biological effect through patient immune monitoring. Leukemia. 2012;26:1681–1684. doi: 10.1038/leu.2011.384. [DOI] [PubMed] [Google Scholar]
- 99.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 100.Xu L, Meng F, Ni M, Lee Y, Li G. N-cadherin regulates osteogenesis and migration of bone marrow-derived mesenchymal stem cells. Mol Biol Rep. 2012 doi: 10.1007/s11033-012-2334-0. [DOI] [PubMed] [Google Scholar]
- 101.Theisen CS, Wahl JK, 3rd, Johnson KR, Wheelock MJ. NHERF links the N-cadherin/catenin complex to the platelet-derived growth factor receptor to modulate the actin cytoskeleton and regulate cell motility. Mol Biol Cell. 2007;18:1220–1232. doi: 10.1091/mbc.E06-10-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Myers TJ, Granero-Molto F, Longobardi L, Li T, Yan Y, Spagnoli A. Mesenchymal stem cells at the intersection of cell and gene therapy. Expert Opin Biol Ther. 2010;10:1663–1679. doi: 10.1517/14712598.2010.531257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bartosh TJ, Ylostalo JH, Mohammadipoor A, Bazhanov N, Coble K, Claypool K, Lee RH, Choi H, Prockop DJ. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A. 2010;107:13724–13729. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alimperti S, Lei P, Wen Y, Tian J, Campbell AM, Andreadis ST. Serum-free spheroid suspension culture maintains mesenchymal stem cell proliferation and differentiation potential. Biotechnology progress. 2014;30:974–983. doi: 10.1002/btpr.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Agarwal SK, Brenner MB. Role of adhesion molecules in synovial inflammation. Curr Opin Rheumatol. 2006;18:268–276. doi: 10.1097/01.bor.0000218948.42730.39. [DOI] [PubMed] [Google Scholar]
- 106.Chang SK, Noss EH, Chen M, Gu Z, Townsend K, Grenha R, Leon L, Lee SY, Lee DM, Brenner MB. Cadherin-11 regulates fibroblast inflammation. Proc Natl Acad Sci U S A. 2011;108:8402–8407. doi: 10.1073/pnas.1019437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ferrari SL, Traianedes K, Thorne M, Lafage-Proust MH, Genever P, Cecchini MG, Behar V, Bisello A, Chorev M, Rosenblatt M, Suva LJ. A role for N-cadherin in the development of the differentiated osteoblastic phenotype. J Bone Miner Res. 2000;15:198–208. doi: 10.1359/jbmr.2000.15.2.198. [DOI] [PubMed] [Google Scholar]
- 108.Cheng SL, Lecanda F, Davidson MK, Warlow PM, Zhang SF, Zhang L, Suzuki S, St John T, Civitelli R. Human osteoblasts express a repertoire of cadherins, which are critical for BMP-2-induced osteogenic differentiation. J Bone Miner Res. 1998;13:633–644. doi: 10.1359/jbmr.1998.13.4.633. [DOI] [PubMed] [Google Scholar]
- 109.Suva LJ, Towler DA, Harada S, Gaub MP, Rodan GA. Characterization of retinoic acid- and cell-dependent sequences which regulate zif268 gene expression in osteoblastic cells. Mol Endocrinol. 1994;8:1507–1520. doi: 10.1210/mend.8.11.7877619. [DOI] [PubMed] [Google Scholar]
- 110.Debiais F, Lemonnier J, Hay E, Delannoy P, Caverzasio J, Marie PJ. Fibroblast growth factor-2 (FGF-2) increases N-cadherin expression through protein kinase C and Src-kinase pathways in human calvaria osteoblasts. J Cell Biochem. 2001;81:68–81. doi: 10.1002/1097-4644(20010401)81:1<68::aid-jcb1024>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 111.Luegmayr E, Glantschnig H, Varga F, Klaushofer K. The organization of adherens junctions in mouse osteoblast-like cells (MC3T3-E1) and their modulation by triiodothyronine and 1,25-dihydroxyvitamin D3. Histochem Cell Biol. 2000;113:467–478. doi: 10.1007/s004180000152. [DOI] [PubMed] [Google Scholar]
- 112.Lecanda F, Cheng SL, Shin CS, Davidson MK, Warlow P, Avioli LV, Civitelli R. Differential regulation of cadherins by dexamethasone in human osteoblastic cells. J Cell Biochem. 2000;77:499–506. doi: 10.1002/(sici)1097-4644(20000601)77:3<499::aid-jcb14>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 113.Marie PJ. Role of N-cadherin in bone formation. J Cell Physiol. 2002;190:297–305. doi: 10.1002/jcp.10073. [DOI] [PubMed] [Google Scholar]
- 114.Kii I, Amizuka N, Shimomura J, Saga Y, Kudo A. Cell-cell interaction mediated by cadherin-11 directly regulates the differentiation of mesenchymal cells into the cells of the osteo-lineage and the chondro-lineage. J Bone Miner Res. 2004;19:1840–1849. doi: 10.1359/JBMR.040812. [DOI] [PubMed] [Google Scholar]
- 115.Kawaguchi J, Azuma Y, Hoshi K, Kii I, Takeshita S, Ohta T, Ozawa H, Takeichi M, Chisaka O, Kudo A. Targeted disruption of cadherin-11 leads to a reduction in bone density in calvaria and long bone metaphyses. J Bone Miner Res. 2001;16:1265–1271. doi: 10.1359/jbmr.2001.16.7.1265. [DOI] [PubMed] [Google Scholar]
- 116.Di Benedetto A, Watkins M, Grimston S, Salazar V, Donsante C, Mbalaviele G, Radice GL, Civitelli R. N-cadherin and cadherin 11 modulate postnatal bone growth and osteoblast differentiation by distinct mechanisms. J Cell Sci. 2010;123:2640–2648. doi: 10.1242/jcs.067777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tuan RS. Cellular signaling in developmental chondrogenesis: N-cadherin, Wnts, and BMP-2. J Bone Joint Surg Am. 2003;85-A(Suppl 2):137–141. doi: 10.2106/00004623-200300002-00019. [DOI] [PubMed] [Google Scholar]
- 118.Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97:33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 119.Quintana L, zur Nieden NI, Semino CE. Morphogenetic and regulatory mechanisms during developmental chondrogenesis: new paradigms for cartilage tissue engineering. Tissue Eng Part B Rev. 2009;15:29–41. doi: 10.1089/ten.teb.2008.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Oberlender SA, Tuan RS. Spatiotemporal profile of N-cadherin expression in the developing limb mesenchyme. Cell Adhes Commun. 1994;2:521–537. doi: 10.3109/15419069409014216. [DOI] [PubMed] [Google Scholar]
- 121.Woods A, Wang G, Dupuis H, Shao Z, Beier F. Rac1 signaling stimulates N-cadherin expression, mesenchymal condensation, and chondrogenesis. J Biol Chem. 2007;282:23500–23508. doi: 10.1074/jbc.M700680200. [DOI] [PubMed] [Google Scholar]
- 122.Nakazora S, Matsumine A, Iino T, Hasegawa M, Kinoshita A, Uemura K, Niimi R, Uchida A, Sudo A. The cleavage of N-cadherin is essential for chondrocyte differentiation. Biochem Biophys Res Commun. 2010;400:493–499. doi: 10.1016/j.bbrc.2010.08.070. [DOI] [PubMed] [Google Scholar]
- 123.Tufan AC, Tuan RS. Wnt regulation of limb mesenchymal chondrogenesis is accompanied by altered N-cadherin-related functions. FASEB J. 2001;15:1436–1438. doi: 10.1096/fj.00-0784fje. [DOI] [PubMed] [Google Scholar]
- 124.Tufan AC, Daumer KM, DeLise AM, Tuan RS. AP-1 transcription factor complex is a target of signals from both WnT-7a and N-cadherin-dependent cell-cell adhesion complex during the regulation of limb mesenchymal chondrogenesis. Exp Cell Res. 2002;273:197–203. doi: 10.1006/excr.2001.5448. [DOI] [PubMed] [Google Scholar]
- 125.Tufan AC, Daumer KM, Tuan RS. Frizzled-7 and limb mesenchymal chondrogenesis: effect of misexpression and involvement of N-cadherin. Dev Dyn. 2002;223:241–253. doi: 10.1002/dvdy.10046. [DOI] [PubMed] [Google Scholar]
- 126.Luo Y, Kostetskii I, Radice GL. N-cadherin is not essential for limb mesenchymal chondrogenesis. Dev Dyn. 2005;232:336–344. doi: 10.1002/dvdy.20241. [DOI] [PubMed] [Google Scholar]
- 127.Shin CS, Lecanda F, Sheikh S, Weitzmann L, Cheng SL, Civitelli R. Relative abundance of different cadherins defines differentiation of mesenchymal precursors into osteogenic, myogenic, or adipogenic pathways. J Cell Biochem. 2000;78:566–577. [PubMed] [Google Scholar]
- 128.Kawaguchi J, Kii I, Sugiyama Y, Takeshita S, Kudo A. The transition of cadherin expression in osteoblast differentiation from mesenchymal cells: consistent expression of cadherin-11 in osteoblast lineage. J Bone Miner Res. 2001;16:260–269. doi: 10.1359/jbmr.2001.16.2.260. [DOI] [PubMed] [Google Scholar]
- 129.Borghi N, James Nelson W. Intercellular adhesion in morphogenesis: molecular and biophysical considerations. Curr Top Dev Biol. 2009;89:1–32. doi: 10.1016/S0070-2153(09)89001-7. [DOI] [PubMed] [Google Scholar]
- 130.Rougon G, Hobert O. New insights into the diversity and function of neuronal immunoglobulin superfamily molecules. Annu Rev Neurosci. 2003;26:207–238. doi: 10.1146/annurev.neuro.26.041002.131014. [DOI] [PubMed] [Google Scholar]
- 131.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730–2741. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol. 2001;159:1009–1020. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hinz B, Gabbiani G. Mechanisms of force generation and transmission by myofibroblasts. Curr Opin Biotechnol. 2003;14:538–546. doi: 10.1016/j.copbio.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 134.Hinz B, Pittet P, Smith-Clerc J, Chaponnier C, Meister JJ. Myofibroblast development is characterized by specific cell-cell adherens junctions. Mol Biol Cell. 2004;15:4310–4320. doi: 10.1091/mbc.E04-05-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schneider DJ, Wu M, Le TT, Cho SH, Brenner MB, Blackburn MR, Agarwal SK. Cadherin-11 contributes to pulmonary fibrosis: potential role in TGF-beta production and epithelial to mesenchymal transition. FASEB J. 2012;26:503–512. doi: 10.1096/fj.11-186098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kovacs EM, Goodwin M, Ali RG, Paterson AD, Yap AS. Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Curr Biol. 2002;12:379–382. doi: 10.1016/s0960-9822(02)00661-9. [DOI] [PubMed] [Google Scholar]
- 137.Noren NK, Niessen CM, Gumbiner BM, Burridge K. Cadherin engagement regulates Rho family GTPases. J Biol Chem. 2001;276:33305–33308. doi: 10.1074/jbc.C100306200. [DOI] [PubMed] [Google Scholar]
- 138.Kovacs EM, Ali RG, McCormack AJ, Yap AS. E-cadherin homophilic ligation directly signals through Rac and phosphatidylinositol 3-kinase to regulate adhesive contacts. J Biol Chem. 2002;277:6708–6718. doi: 10.1074/jbc.M109640200. [DOI] [PubMed] [Google Scholar]
- 139.Brieva TA, Moghe PV. Engineering the hepatocyte differentiation-proliferation balance by acellular cadherin micropresentation. Tissue Eng. 2004;10:553–564. doi: 10.1089/107632704323061915. [DOI] [PubMed] [Google Scholar]
- 140.Brieva TA, Moghe PV. Exogenous cadherin microdisplay can interfere with endogenous signaling and reprogram gene expression in cultured hepatocytes. Biotechnol Bioeng. 2004;85:283–292. doi: 10.1002/bit.10855. [DOI] [PubMed] [Google Scholar]
- 141.Lambert M, Padilla F, Mege RM. Immobilized dimers of N-cadherin-Fc chimera mimic cadherin-mediated cell contact formation: contribution of both outside-in and inside-out signals. J Cell Sci. 2000;113(Pt 12):2207–2219. doi: 10.1242/jcs.113.12.2207. [DOI] [PubMed] [Google Scholar]
- 142.Charrasse S, Meriane M, Comunale F, Blangy A, Gauthier-Rouviere C. N-cadherin-dependent cell-cell contact regulates Rho GTPases and beta-catenin localization in mouse C2C12 myoblasts. J Cell Biol. 2002;158:953–965. doi: 10.1083/jcb.200202034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gavard J, Marthiens V, Monnet C, Lambert M, Mege RM. N-cadherin activation substitutes for the cell contact control in cell cycle arrest and myogenic differentiation: involvement of p120 and beta-catenin. J Biol Chem. 2004;279:36795–36802. doi: 10.1074/jbc.M401705200. [DOI] [PubMed] [Google Scholar]
- 144.Evans SF, Docheva D, Bernecker A, Colnot C, Richter RP, Knothe Tate ML. Solid-supported lipid bilayers to drive stem cell fate and tissue architecture using periosteum derived progenitor cells. Biomaterials. 2013;34:1878–1887. doi: 10.1016/j.biomaterials.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 145.Pittet P, Lee K, Kulik AJ, Meister JJ, Hinz B. Fibrogenic fibroblasts increase intercellular adhesion strength by reinforcing individual OB-cadherin bonds. J Cell Sci. 2008;121:877–886. doi: 10.1242/jcs.024877. [DOI] [PubMed] [Google Scholar]
- 146.Lira CB, Chu K, Lee YC, Hu MC, Lin SH. Expression of the extracellular domain of OB-cadherin as an Fc fusion protein using bicistronic retroviral expression vector. Protein Expr Purif. 2008;61:220–226. doi: 10.1016/j.pep.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang Y, Zhou Y, Zhu J, Dong S, Li C, Xiang Q. Effect of a novel recombinant protein of fibronectinIII7–10/cadherin 11 EC1–2 on osteoblastic adhesion and differentiation. Biosci Biotechnol Biochem. 2009;73:1999–2006. doi: 10.1271/bbb.90187. [DOI] [PubMed] [Google Scholar]
- 148.Griffin MA, Sen S, Sweeney HL, Discher DE. Adhesion-contractile balance in myocyte differentiation. J Cell Sci. 2004;117:5855–5863. doi: 10.1242/jcs.01496. [DOI] [PubMed] [Google Scholar]
- 149.Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 151.Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chin VI, Taupin P, Sanga S, Scheel J, Gage FH, Bhatia SN. Microfabricated platform for studying stem cell fates. Biotechnol Bioeng. 2004;88:399–415. doi: 10.1002/bit.20254. [DOI] [PubMed] [Google Scholar]
- 153.Tang J, Peng R, Ding J. The regulation of stem cell differentiation by cell-cell contact on micropatterned material surfaces. Biomaterials. 2010;31:2470–2476. doi: 10.1016/j.biomaterials.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 154.Wang X, Song W, Kawazoe N, Chen G. The osteogenic differentiation of mesenchymal stem cells by controlled cell-cell interaction on micropatterned surfaces. J Biomed Mater Res A. 2013 doi: 10.1002/jbm.a.34645. [DOI] [PubMed] [Google Scholar]
- 155.Gray DS, Liu WF, Shen CJ, Bhadriraju K, Nelson CM, Chen CS. Engineering amount of cell-cell contact demonstrates biphasic proliferative regulation through RhoA and the actin cytoskeleton. Exp Cell Res. 2008;314:2846–2854. doi: 10.1016/j.yexcr.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nelson CM, Liu WF, Chen CS. Manipulation of cell-cell adhesion using bowtie-shaped microwells. Methods Mol Biol. 2007;370:1–10. doi: 10.1007/978-1-59745-353-0_1. [DOI] [PubMed] [Google Scholar]
- 157.Charest JL, Jennings JM, King WP, Kowalczyk AP, Garcia AJ. Cadherin-mediated cell-cell contact regulates keratinocyte differentiation. J Invest Dermatol. 2009;129:564–572. doi: 10.1038/jid.2008.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tian J, Andreadis ST. Independent and high-level dual-gene expression in adult stem-progenitor cells from a single lentiviral vector. Gene Ther. 2009;16:874–884. doi: 10.1038/gt.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Alimperti S, Lei P, Tian J, Andreadis ST. A novel lentivirus for quantitative assessment of gene knockdown in stem cell differentiation. Gene therapy. 2012;19:1123–1132. doi: 10.1038/gt.2011.208. [DOI] [PubMed] [Google Scholar]
- 160.Padmashali RM, Andreadis ST. Engineering fibrinogen-binding VSV-G envelope for spatially- and cell-controlled lentivirus delivery through fibrin hydrogels. Biomaterials. 2011;32:3330–3339. doi: 10.1016/j.biomaterials.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 161.Raut SD, Lei P, Padmashali RM, Andreadis ST. Fibrin-mediated lentivirus gene transfer: implications for lentivirus microarrays. J Control Release. 2010;144:213–220. doi: 10.1016/j.jconrel.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tian J, Alimperti S, Lei P, Andreadis ST. Lentiviral microarrays for real-time monitoring of gene expression dynamics. Lab on a chip. 2010;10:1967–1975. doi: 10.1039/c003153d. [DOI] [PubMed] [Google Scholar]
- 163.Padmashali RM, Mistriotis P, Liang MS, Andreadis ST. Lentiviral arrays for live-cell dynamic monitoring of gene and pathway activity during stem cell differentiation. Mol Ther. 2014;22:1971–1982. doi: 10.1038/mt.2014.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Moharil J, Lei P, Gaile D, Andreadis ST. Lentiviral microarrays for high throughput monitoring MSC differentiation along the myogenic lineage. 2014. submitted. [Google Scholar]