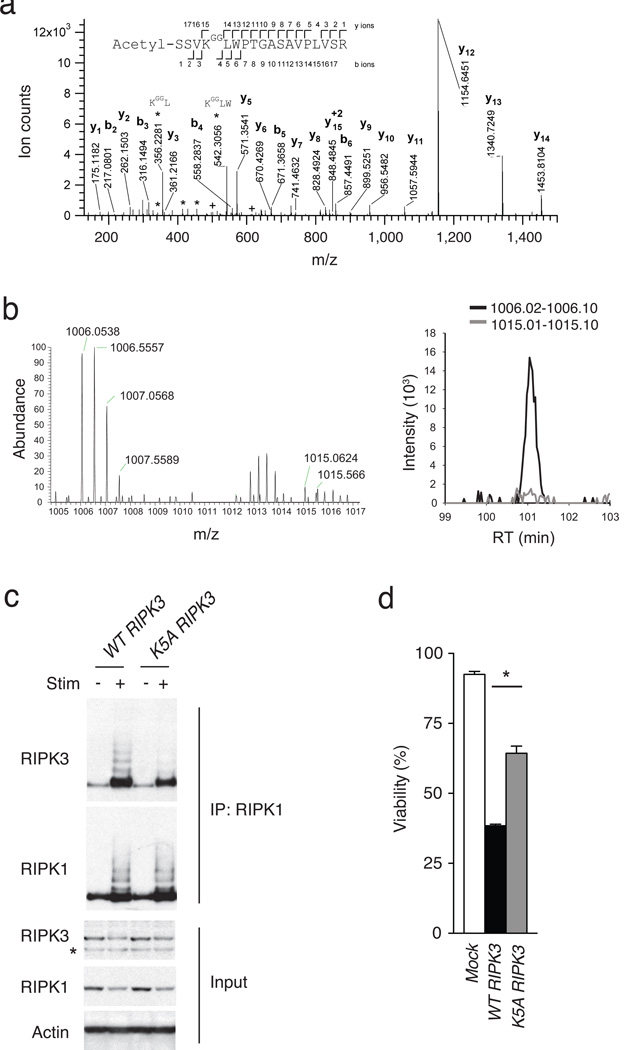
Figure 5. RIPK3 ubiquitination supports RIPK1-RIPK3 complexes and necroptosis.
(a) Site specific ubiquitination of RIPK3. A20−/− MEFs were stimulated with TNF, CHX and ZVAD, and lysed after 4 hours. Lysates were immunoprecipitated with anti-gly-gly antibody, after which immunoprecipitates were analyzed by nanoflow-UPLC-HCD-MSMS. A HCD tandem mass spectrum obtained from a precursor ion with m/z value 1006.0525+2 from A20−/− cells undergoing necroptosis is shown. A data base search showed the spectra to identify the peptide spanning amino acids 2 to 19 of RIPK3, acetylated in the N terminus and carrying a di-glycine remnant on K5 (theoretical monoisotopic m/z= 1006.0522+2). Sequence ions are labeled in the figure, and indicated with marks over (C-terminal ions) and below (N-terminal ions) the sequence of the peptide. Prominent ions corresponding to internal fragments are labeled (*). Ions labeled (+) show fragments with masses 501.2667 and 614.3508, corresponding to ions b4 and b5 undergoing a second fragmentation, with a loss of 57.02146 mass units, corresponding to the terminal glycine on the ɛ-amino of RIPK3 K5. (b) SILAC based mass spectrometric quantitation of RIPK3 ubiquitination. Heavy (A20+/+) and light (A20−/−) isotope labeled MEFs were stimulated to undergo necroptosis, after which cell lysates were mixed and then analysed by ubiscan assisted mass spectrometry as in (a). The di-glycine remnant of RIPK3 K5 from light isotope labeled cells (A20−/− cells) was again identified at m/z = 1006, while the heavy isotope labeled cells (A20+/+ cells) contained the RIPK3 K5 peptide at m/z = 1015 (spectra on left). The relative abundance of these two forms of the ubiquitinated K5 peptide is displayed in the histogram at right. (c) K5 ubiquitination supports RIPK1-RIPK3 complexes. WT or mutant K5A RIPK3 expression constructs were transduced into A20−/− RIPK3−/− MEFs using a GFP-expressing lentivirus, after which cells were FACS-sorted to obtain pure populations of transduced (GFP+) cells. Cells were then stimulated for four hours, lysed and analysed for RIPK1-associated RIPK3 expression by immunoblotting. “+” indicates treatment with TNF, CHX, and ZVAD; “-” indicates media control. RIPK1 immunoblot of RIPK1 IP is shown below RIPK3 immunoblot as control for IP; immunoblots for RIPK3, RIPK1, and actin on whole cell lysates are shown below as controls. * indicates non-specific band below specific RIPK3 band. (d) K5 ubiquitination supports necroptosis. A20−/− Ripk3−/− MEFs complemented with RIPK3 constructs in (c) above analyzed for sensitivity to necroptosis. Survival of MEFs expressing the indicated forms of RIPK3 is shown relative to unstimulated cells. Bars indicate standard deviation. * indicates p < 0.05 when compared with cells expressing WT RIPK3.