Abstract
Pseudomonas aeruginosa and Streptococcus pneumoniae are causative agents in a wide range of infections. Genes encoding proteins corresponding to phenylalanyl-tRNA synthetase (PheRS) were cloned from both bacteria. The two forms of PheRS were kinetically evaluated and the Km’s for P. aeruginosa PheRS with its three substrates, phenylalanine, ATP and tRNAPhe were determined to be 48, 200, and 1.2 μM, respectively, while the Km’s for S. pneumoniae PheRS with respect to phenylalanine, ATP and tRNAPhe were 21, 225 and 0.94 μM, respectively. P. aeruginosa and S. pneumoniae PheRS were used to screen a natural compound library and a single compound was identified that inhibited the function of both enzymes. The compound inhibited P. aeruginosa and S. pneumoniae PheRS with IC50’s of 2.3 and 4.9 μM, respectively. The compound had a Ki of 0.83 and 0.98 μM against P. aeruginosa and S. pneumoniae PheRS, respectively. The minimum inhibitory concentration (MIC) of the compound was determined against a panel of Gram positive and negative bacteria including efflux pump mutants and hyper-sensitive strains. MICs against wild-type P. aeruginosa and S. pneumoniae cells in culture were determined to be 16 and 32 μg/ml, respectively. The mechanism of action of the compound was determined to be competitive with the amino acid, phenylalanine, and uncompetitive with AT P. There was no inhibition of cytoplasmic protein synthesis, however, partial inhibition of the human mitochondrial PheRS was observed.
Keywords: Drug discovery, natural product, protein synthesis, Pseudomonas, Streptococcus
INTRODUCTION
The Gram-negative microorganism Pseudomonas aeruginosa, an opportunistic bacterial pathogen, is the causative agent in a wide range of infections. P. aeruginosa is responsible for over one-seventh of all nosocomial infections, with strains that are multidrug-resistant becoming increasingly common [1,2]. Clinical isolates of antimicrobial resistance strains of P. aeruginosa are significant and growing [3] and have become a leading problem in the hospital setting [4]. However, the primary medical problem resulting from P. aeruginosa infections are lung colonization associated with cystic fibrosis [5] in which the chronic infections are the main causes of patient morbidity and mortality [6].
Streptococcus pneumoniae, a Gram-positive bacterium, is the most common cause of bacteremia, pneumonia, meningitis, otitis media and other invasive diseases [7] and a primary cause of upper respiratory tract infections in children [8]. The recent explosion of multi-drug resistance among S. pneumoniae is increasing worldwide [9]. Resistance to betalactams, macrolides and fluoroquinolone is now common in S. pneumoniae [10]. The Assessing Worldwide Antimicrobial Resistance Evaluation (AWARE) surveillance program recently concluded that multi-drug resistance was greater than 30% in S. pneumoniae [11,12].
Aminoacyl-tRNA synthetases (aaRS) are potential targets for the development of new antibiotics designed to combat isolates resistant to many antibiotics currently in use. The aaRS are a class of enzymes which catalyze the covalent esterification of an amino acid to its cognate tRNAs during protein biosynthesis. There are two major classes of aaRSs which are based on distinct structural active site regions. The class I aaRS contains a characteristic ATP binding motif, the Rossmann fold, while three structural motifs compose the ATP binding site in class II aaRS [13]. In the class II aaRS, motif 1 appears to be critical for subunit association, and motifs 2 and 3 form parts of the active site during the aminoacylation reaction [14]. The class II aaRSs are further divided into three subgroups: class IIa, class IIb and class IIc. Members of class IIa and class IIc aaRSs are characterized by the lack of a specific C-terminal domain and the absence of a specific N-terminal domain, respectively, that are characteristic of the subgroup class IIb [15]. Phenylalanyl-tRNA synthetase (PheRS) is in the class IIc subgroup aaRSs which also includes AlaRS and GlyRS. PheRS and GlyRS are formed as α2β2 tetramers in most systems and AlaRS is an α4 tetramer [16]. All of the subunits are required for aminoacylation of the cognate tRNA [17]. The crystal structure of Thermus thermophilus PheRS indicates that the tetrameric form of the enzyme is functional as an (αβ)2 structure, that is a dimer constructed from two heterodimers. Two tRNA molecules are bound by this complex and each of the four subunits in the complex interacts with the three other subunit and with the two bound tRNA substrates [18,19]. Each α-subunit primarily interacts with the CCA acceptor end of the proximal tRNA but recognizes the anticodon stem and loop of the tRNA bound by the distal αβ subunits [18].
Aminoacyl-tRNA synthetases are essential enzymes in protein biosynthesis and individually are attractive targets for the discovery of antibiotics [20]. We describe here the cloning and enzymatic characterization of PheRS from P. aeruginosa and S. pneumoniae and use of high throughput screening (HTS) systems for the detection of compounds that inhibit the activities of these enzymes. Using these systems, we have screened over 300 natural compounds for inhibitory activity against both of these synthetases. Natural products testing in this area are novel and are under represented in screening in general.
METHODS AND MATERIALS
Materials
Oligonucleotides were purchased from the Integrated DNA Technologies (Coralville, IA). All other materials were purchased from Sigma Aldrich (St. Louis, MO) or Fisher Scientific (Pittsburg, PA). DNA sequencing was carried out at the Howard Hughes Medical Institute (HHMI) laboratory at The University of Texas – Pan American and Functional Bioscience (Madison, WI). Radioactive isotopes were from PerkinElmer (Waltham, MA). Moraxella catarrhalis (ATCC 25238), Enterococcus faecalis (ATCC 29212), Streptococcus pneumonia (ATCC 49619), Escherichia coli (ATCC 25922), Staphylococcus aureus (ATCC 29213), Haemophilus influenza (ATCC 49766), Pseudomonas aeruginosa (ATCC 47085) were from the American Type Culture Collection (Manassas, VA). E. coli tolC mutant, P. aeruginosa PAO200 (efflux pump mutant) and P. aeruginosa hypersensitive strain (ATCC 35151) were a kind gift from Urs Ochsner (Crestone Pharma-Boulder CO). Human mitochondrial PheRS (hmPheRS) was prepared as described [21]. Chemical compounds were from Prestwick Chemical (Illkirch, France).
Gel Electrophoresis and Protein Analysis
SDS-PAGE was performed using 4-12% polyacrylamide pre-cast gels (Novex NuPAGE; Invitrogen) with MOPS running buffer (Invitrogen). EZ-Run™ Rec Protein Ladder was from Fisher. Gels were stained with SimplyBlue Safe Stain (Invitrogen). Protein concentrations were determined using protocols from Bradford [22] using bovine serum albumin as the standard. Protein alignments were prepared using Vector NTI Advance ® (Invitrogen).
Cloning and Purification of P. aeruginosa and S. pneumoniae PheRS
The genes encoding the α and β subunits of P. aeruginosa PheRS were amplified by PCR (Bio-Rad MJ Mini Thermo Cycler) from P. aeruginosa PAO1 (ATCC 47085) genomic DNA. The genes encoding the α (pheS) and β (pheT) subunits were cloned as a natural operon using a forward primer (5’-ctgagctagcgaaaacctggatgcgctg-3’) designed to add an NheI restriction site to the 5’ end of the operon and a reverse primer (5’-gactaagcttctactatttccttaacgtggcattg-3’) which was designed to add a HindIII restriction site to the 3’ end of the operon. The PCR product was inserted into a pET-28b(+) plasmid (Novagen) digested with NheI/ HindIII. This placed the gene encoding the α-subunit downstream of a sequence encoding six histidine residues. The recombinant plasmid was subsequently transformed into Rosetta 2(DE3) competent cells (Novagen).
The genes encoding the S. pneumoniae PheRS α and β subunits of PheRS were amplified individually from S. pneumoniae genomic DNA (ATCC 49619). The gene encoding the α subunit was amplified using a forward primer (5’-gactcatatgtcaactattgaagaacaattaaaa-3’) designed to add an NdeI restriction site to the 5’ end of the gene and a reverse primer (5’-agttggatccttatttaaactgttctgagaagcg-3’) which was designed to add a BamHI restriction site to the 3’ end of the gene. The PCR product was inserted into a pET-28b(+) plasmid (Novagen) digested with NdeI/ BamHI. The gene encoding the β subunit was amplified using a forward primer (5’-gactcatatgcttgtatcttataaatggttaaaa-3’) designed to add an NdeI restriction site to the 5’ end of the gene and a reverse primer (5’-gacactcgagacgcacttctgcattgactt-3’) which was designed to add an XhoI restriction site to the 3’ end of the gene. The PCR product was inserted into pET-24b(+) plasmid (Novagen) digested with NdeI/XhoI. Both recombinant plasmids were transformed individually into Rosetta 2(DE3) competent cells (Novagen).
Bacterial cultures were grown in Terrific Broth containing 25 μg/mL of kanamycin and 50 μg/mL of chloramphenicol. Cultures expressing P. aeruginosa PheRS α and β subunits were grown at 37 ºC and the target proteins were expressed at an optical density (A600) of 0.6-0.8 by the addition of isopropyl β3-D-1-thiogalactopyranoside (IPTG) to 0.5 mM. Cultures expressing S. pneumoniae α and β subunits were grown to an optical density (A600) of 0.6-0.8 at 37 °C, the temperature was then lowered to 30 °C and target protein expression was induced by the addition of IPTG to 0.25 mM. Growth of all bacterial cultures was continued for 2 h post-induction, and the bacteria were harvested by centrifugation (10000 ×g, 30 min, 4 °C).
To purify the target proteins, bacterial cells were lysed and fraction I lysate was prepared as previously described [23]. P. aeruginosa PheRS was precipitated by the addition of ammonium sulfate (AS) to 50% saturation and S. pneumoniae α and β subunits were precipitated at 35% and between 45 and 60% AS saturation, respectively. The precipitated proteins were collected by centrifugation (23,000 × g, 60 min, 4 °C). The target proteins were further purified to greater than 98% homogeneity using nickel-nitrilotriacetic acid (NTA) affinity chromatography (Perfect Pro, 5 Prime) followed by dialysis (two times) against a buffer containing: 20 mM Hepes-KOH (pH 7.0), 40 mM KCl, 1 mM MgCl2, 0.1 mM EDTA, 10 % glycerol. S. pneumoniae α and β subunits were combined at a molar ratio of 1:1. Finally, all proteins were fast frozen in liquid nitrogen and stored at −80 °C.
ATP:PPi Exchange Reactions
ATP:PPi exchange reactions (100 μl) were carried out at 37 °C in 50 mM Tris-HCl (pH 7.5), 10 mM KF, 8 mM MgCl2, 1 mM dithiothreitol (DTT), 2 mM [32P]PPi (50 cpm/pmol) and contained 0.1 μM of either S. pneumoniae or P. aeruginosa PheRS. In reactions in which the concentration of ATP was varied, the amino acid phenylalanine (Phe) remained constant at 2 mM; alternatively, when the concentration of Phe was varied, the ATP concentration remained at 2 mM. The exchange of PPi in these reactions was measured between one and five min. Reactions were stopped by diluting 5 μl of the reaction mix into 45 μl of a buffer containing 20 mM Hepes-KOH (pH 7.0), 40 mM KCl, 1 mM MgCl2, 0.1 mM EDTA, 10% glycerol and immediately spotting 5 μl of the diluted solution onto PEI cellulose TLC plates (Selecto Scientific). ATP and PPi were separated using a solution containing 4 M urea and 0.75 M KPi (pH 3.5) as a mobile phase [24]. Amounts of ATP, PPi and Pi were quantified using a Typhoon FLA 7000 Laser Scanner (GE Healthcare). Initial velocities for exchange of PPi were determined and the kinetic parameters (KM and Vmax) for the interactions of PheRS with ATP and phenylalanine were determined by plotting the velocities against substrate concentration and fit to the Michaelis-Menten steady-state model using XLfit (IDBS).
tRNA Aminoacylation Assays
All of the aminoacylation reactions were at 37 °C and at time intervals between one and five minutes. Reactions (50 μl) contained 50 mM Tris-HCl (pH 7.5), 8 mM MgCl2, 1.25 mM ATP, 1 mM spermine, 1 mM DTT, 100 μM [3H]Phe (75 cpm/pmol), and 6.25 nM S. pneumoniae or P. aeruginosa PheRS. The tRNA was from E. coli MRE600 (Roche). The tRNAPhe concentrations were varied as indicated. Reactions were stopped by the addition of 2 ml of 5% (v/v) ice-cold trichloroacetic acid (TCA), placed on ice for ten min, then filtered through glass fiber filters (Millipore, type HA 0.45 mm). Filters were washed with 10 ml ice-cold 5% TCA, dried and counted in an LS6500 multi-purpose scintillation counter (Beckman Coulter). Initial velocities for aminoacylation were calculated for all tRNA concentrations. These data were modeled using the Michaelis Menten equation as described above and the KM and Vmax values were determined.
Chemical Compound Screening
The natural product compound library (320 compounds) was screened using the tRNA aminoacylation assay adapted to a scintillation proximity assay (SPA) [25]. The chemical compounds were screened using 96-well microtiter plates (Costar). Test compounds dissolved in 100% DMSO (2 μl of compound; 3.3 mM) were equilibrated by the addition of 33 μl of the protein/substrate mix described above for the aminoacylation assay (minus tRNA). Control reactions contained only DMSO with no compound. The concentration of P. aeruginosa PheRS was 0.05 µM and S. pneumoniae PheRS was 0.1 µM. This mixture was incubated at ambient temperature for 15 min and then reactions were started by the addition of 15 μl E. coli tRNA (80 μM total tRNA or 2 μM tRNAPhe), followed by incubation for 1 h at 37 °C. Reactions were stopped by the addition of 5 µl of 0.5 M EDTA. 400 μg of yttrium silicate (Ysi) poly-L-lysine coated SPA beads (Perkin-Elmer) in 150 μl of 300 mM citric acid were added and allowed to incubate at room temperature for 1 h. The plates were analyzed using a 1450 Microbeta (Jet) liquid scintillation/luminescent counter (Wallac). Assays to determine IC50 values were as described with the test compounds serially diluted from 200 μM to 0.4 μM.
Microbiological Assays
Broth microdilution MIC testing was carried out in 96-well microtiter plates according to Clinical Laboratory Standards Institute guideline M7-A7 [26]. MIC values were determined for E. coli (ATCC 25922), E. coli tolC mutant, Enterococcus faecalis (ATCC 29212), Haemophilus influenzae (ATCC 49766), Moraxella catarrhalis (ATCC 25238), P. aeruginosa (ATCC 47085), P. aeruginosa PAO200 (efflux pump mutant), P. aeruginosa hypersensitive strain (ATCC 35151), S. aureus (ATCC 29213), and S. pneumonia (ATCC 49619) from the American Type Culture Collection (Manassas, VA). For quality control (QC) of MIC determination and culture purity, MICs were determined for antibiotics specific for each bacterial strain. The antibiotic and concentration range for each bacteria was: E. coli (ampicillin, 0.125-128 μg/ml), E. faecalis (vancomycin 0.125-128 μg/ml), H. influenzae (ampicillin, 0.03125-32 μg/ml), M. catarrhalis (ampicillin, 0.015625-16 μg/ml), P. aeruginosa (ampicillin, 0.125-128 μg/ml), S. aureus (oxacillin, 0.03125-32 μg/ml), S. pneumoniae (penicillin, 0.0625-64 μg/ml).
Eukaryotic Assays
Determination of the effect of inhibitory compound BPP_03B04 on cytoplasmic protein synthesis using wheat germ extracts was as described [27]. To test the effect of BPP_03B04 on the activity of human mitochondrial PheRS (hmPheRS) aminoacylation assays (50 μl) were carried out. The assay mix contained 50 mM Tris-HCl (pH 7.5), 1 mM spermine, 10 mM MgOAc, 2.5 mM ATP, 1 mM DTT, 75 μM Phe, and 0.2 μM hmPheRS. The mix was incubated for 15 min with 132 μM compound, then the reaction was initiated by the addition of tRNAPhe (2 μM). The reaction was stopped by diluting into 2 ml of ice-cold 5% TCA and filtering through glass fiber filters as described above.
Mechanism of Action of Inhibitory Compound
To determine competition with ATP, IC50s were determined in SPA reactions as described above but containing varying ATP concentrations (25, 50, 100, 250, 500, 1000 μM). Final compound concentrations in each IC50 reaction ranged from 200 to 0.4 µM. Positive controls contained only DMSO (2 μl) without compound. To determine competition with Phe the same assay was used. However, ATP was held at a constant concentration of 2.5 mM in assays containing the indicated concentrations of Phe (25, 50, 100, 200, 300 μM).
RESULTS
Sequence Analysis
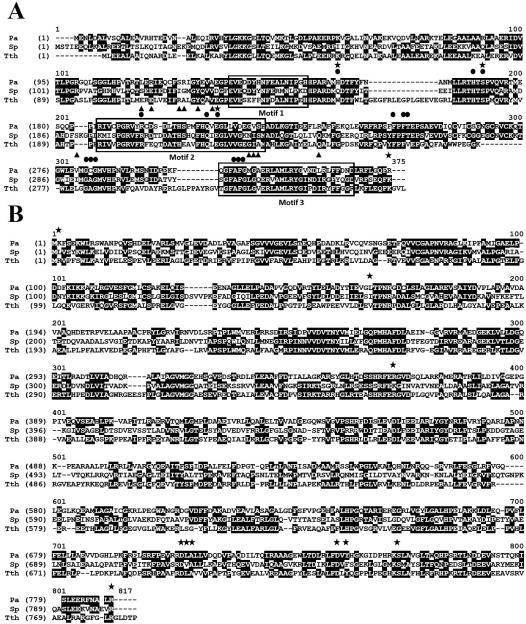
The architecture of the PheRS enzymes has been extensively described, primarily from structural studies of PheRS from T. thermophilus [18,28-30] and more recently the structure of PheRS from E. coli [31], Staphylococcus haemolyticus [32] and P. aeruginosa [33] has been solved. The amino acid sequence conservation when comparing that of T. thermophilus PheRS with the corresponding enzymes from E. coli, P. aeruginosa, or S. pneumoniae is very similar (Table 1). Comparison of the α-subunit from E. coli, P. aeruginosa, S. pneumoniae or S. haemolyticus with the α-subunit of PheRS from T. thermophilus indicates only a small variation in the conservation of the amino acid sequence in which the residues are 53-58% similar and 38-44% identical. There appears to be two regions (α156-170 and α302-310, T. thermophilus numbering) that are present in T. thermophilus PheRS but are not present in P. aeruginosa or S. pneumoniae PheRS (Fig. 1). These are solvent exposed regions on the side of PheRS opposite to that of the bound tRNA and removed from the active site and are unlikely to have an effect on aminoacylation. The amino acid sequence in the β-subunit is only slightly less conserved with a variation of 49-50% similar and 33-36% identical residues. In general, the sequences of functional residues in the subunits of PheRS from T. thermophilus, E. coli, P. aeruginosa, S. pneumoniae or S. haemolyticus are similar containing 73% and 70% conservation of consensus positions in the α- and β-subunits, respectively.
Table 1.
T. thermophilus PheRS amino acid conservation with homologs.
| T. thermophilus PheRS | α-subunit | β-subunit |
|---|---|---|
| E. coli | 53.9/43.8a | 49.3/34.6 |
| P. aeruginosa | 54.3/44.3 | 49.6/36.3 |
| S. pneumoniae | 58.0/44.0 | 50.0/33.6 |
| S. haemolyticus | 52.7/37.8 | 49.3/33.4 |
% similar amino acids/% conserved amino acids
Fig. (1).
An alignment of P. aeruginosa and S. pneumoniae PheRS with PheRS from Thermus thermophilus. The protein sequences were downloaded from the National Center for Biotechnology Information (NCBI). Accession numbers for the a and B subunits of P. aeruginosa (Pa), S. pneumoniae (Sp) and T. thermophilus (Tth) sequences are AAG06128 and AAG06127, ABJ55158 and ACF55813, and WP_011229046 and P27002, respectively. Sequence alignments were performed using Vector NTI Advance (R) 11.5 (Invitrogen). A.) Alignment of the amino acid sequences of a subunits. The three structural motifs are enclosed by boxes. Amino acids that interact with Phe (●), ATP (▲) and tRNA (✩) are indicated. B.) Alignment of the amino acid sequences of B subunits. Amino acids that interact with tRNA (✩) are indicated.
In the α-subunit, active site residues that have been shown to interact with the amino acid substrate, phenylalanine, are almost entirely conserved with only moderate changes observed (Fig. 1). In the T. thermophilus α-subunit, Trp149, which forms hydrogen bonds with Phe, is replaced with the amino acids, glutamine in S. pneumoniae and histidine in P. aeruginosa. Trp149 has also been shown to form hydrogen bonds with the terminal 3’ adenosine of the tRNA [18]. Other moderate replacements occur at Val261 (T. thermophilus numbering) and Ala283. Amino acids in the α-subunit that interacts with ATP, are also well conserved differing only between alanine, leucine, isoleucine and valine except at position 213, in which a glutamic acid in T. thermophilus is replaced by a serine in both S. pneumoniae and P. aeruginosa. The α-subunit also interacts with the acceptor end of the tRNA during the aminoacylation process. In T. thermophilus PheRS, Trp149, Ser180 and Glu220 form hydrogen bonds with the terminal adenosine of the tRNA [18,29]. Of these residues, only Trp149 is not conserved. Phe258 and Phe260 interact with the base of the terminal adenosine in ring-ring interactions and are strictly conserved in the T. thermophilus, P. aeruginosa, and S. pneumoniae α-subunits. In general, functional residues in the active site appear to be well conserved suggesting that the structure of the active site in P. aeruginosa, and S. pneumoniae α-subunit does not diverge significantly from that of other bacteria in which the structure of PheRS has been solved.
In the β-subunit, amino acids at the N-terminus form salt-bridges with C74 and A73 of the acceptor stem of the tRNA. The amino acid at position two varies from an arginine in T. thermophilus PheRS to a lysine and leucine in P. aeruginosa and S. pneumoniae, respectively. Other amino acids that interact with the acceptor stem in the β-subunit are at positions 160 and 362 (T. thermophilus numbering) and only vary from leucine to isoleucine. Amino acids of the β-subunit that act in anticodon recognition are well conserved between the three species and only modest divergences are observed.
Protein Expression and Characterization
The genes encoding the α- and β-subunits of PheRS from P. aeruginosa occur as a natural operon and are translationally coupled. Therefore, these genes were cloned together and inserted into a plasmid that allowed the α-subunit to be expressed fused to an N-terminal peptide containing six histidine residues. The α-subunit was purified using affinity chromatography and the β-subunit was co-purified by association with the α-subunit. The genes encoding the α- and β-subunits of PheRS from S. pneumoniae occur separated by almost 600 nucleotides, therefore each of these genes was cloned separately. The α-subunit was expressed fused to an N-terminal histidine tag and the β-subunit was expressed fused to a C-terminal histidine tag. The mature from of PheRS contains two copies of both α and β subunits, therefore after being expressed and purified separately the two subunits were mixed at a 1:1 molar ratio (Fig. 2).
Fig. (2).
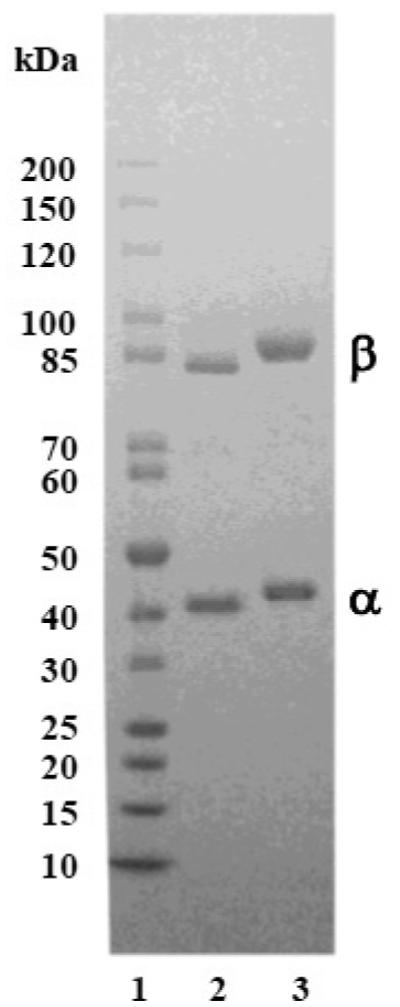
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of purified P. aeruginosa and S. pneumoniae PheRS. Samples (1.5 μg) of both forms of PheRS preparations were analyzed on a 4-20% SDS-PAGE gel and the protein bands were visualized by staining with Coomassie blue. Lane #1, contains protein standard; lane #2 contains P. aeruginosa PheRS; lane #3 contains S. pneumoniae PheRS.
The result of the aminoacylation reaction is the covalent attachment of a specific amino acid to the cognate tRNA and occurs as a two-step process. In the first step, the amino acid and ATP are condensed resulting in the formation of an aminoacyl-adenylate intermediate followed by the release of a pyrophosphate. This reaction is reversible in the absence of a cognate tRNA in most of the aaRS and the parameters governing the kinetic interaction of the aaRS with the amino acid and ATP substrates may be determined. The kinetic parameters KM, kcat and kcat/KM for the interaction of both P. aeruginosa and S. pneumoniae PheRS with the substrates Phe and ATP have been determined by measuring the initial rate of the ATP:PPi exchange. This data was then fit to the Michaelis-Menten steady-state model. Determination of KM and kcat values for Phe was determined at a constant concentration of ATP (2 mM), while the concentration of Phe was varied between 6.25 and 300 μM (Fig. 3A). The KM and kcat values for the interaction with Phe calculated from these data were 48 μM and 16 s−1 for P. aeruginosa PheRS, and 21 μM and 22.5 s−1 for S. pneumoniae PheRS, all respectively (Table 2). E. coli PheRS has a similar KM of 40 μM, but the kcat is significantly higher at 138 s−1. A similar analysis was carried out by varying the concentration of ATP between 100 and 750 μM while maintaining the constant concentration of Phe (2 mM) (Fig. 3B). The KM and kcat values for ATP calculated from these data are 200 μM and 13 s−1 for P. aeruginosa PheRS, and 225 μM and 15.5 s−1 for S. pneumoniae PheRS, all respectively. E. coli PheRS has a KM for ATP (800 μM) that is only 4-fold higher, but the turnover rate (182 s−1) is quite high as compared to that of either P. aeruginosa or S. pneumoniae PheRS.
Fig. (3).
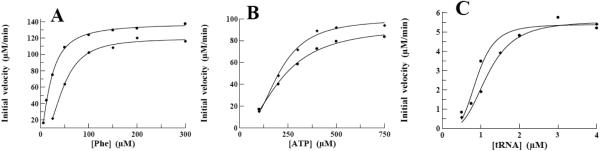
Determination of kinetic parameters for interactions of P. aeruginosa and S. pneumoniae PheRS with the substrates Phe, ATP, and tRNAPhe. Initial velocities for the interaction of P. aeruginosa and S. pneumoniae PheRS with Phe, ATP, and tRNAPhe were determined and the data were fit to a Michaelis-Menten steady-state model using XLfit (IDBS) to determine KM and Vmax. Filled diamonds (◆) represent data collected from assays containing P. aeruginosa PheRS and filled circles (●) represent data collected from assays containing S. pneumoniae PheRS. The graphs contain data from interactions with the substrates A.) Phe, B.) ATP, and C.) tRNAPhe.
Table 2.
Comparison of the kinetic parameters governing the interaction of PheRS with its substrates.
| PheRS | tRNA | Phe | ATP | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
KM
(μM) |
kcat
(s−1) |
kcat/KM
(s−1, μM−1) |
KM
(μM) |
kcat
(s−1) |
kcat/KM
(s−1, μM−1) |
KM
(μM) |
kcat
(s−1) |
kcat/KM
(s−1, μM−1) |
|
| P. aeruginosa | 1.2 | 0.74 | 0.64 | 48 | 16.2 | 0.34 | 200 | 13.2 | 0.066 |
| S. pneumoniae | 0.94 | 0.93 | 1.0 | 21 | 22.5 | 1.1 | 225 | 15.5 | 0.070 |
The formation of an ester bond between the 2’ hydroxyl group (in the case of PheRS) of the 3’ terminal adenosine base of the tRNA and the carboxylic acid function of Phe constitutes the second step in the aminoacylation process. This aminoacylation reaction catalyzed by either P. aeruginosa or S. pneumoniae PheRS was stimulated two-fold by the presence of spermine. The optimal concentration of spermine was observed to be 1-2 mM for both enzymes and the optimal Mg++ concentrations was 7.5 and 10 mM, respectively for P. aeruginosa and S. pneumoniae PheRS. The ability of both P. aeruginosa and S. pneumoniae PheRS to aminoacylate tRNAPhe was determined at various concentrations of tRNA while maintaining ATP and Phe at 2.5 mM and 75 μM, respectively (Fig. 3C). The KM and kcat values were determined to be 1.2 μM and 0.74 s−1 for P. aeruginosa PheRS and 0.94 μM and 0.93 s−1 for S. pneumoniae PheRS. The KM values are 10-fold higher than observed for PheRS from E. coli (0.08 μM) but the turnover numbers are similar to those seen with E. coli PheRS (1.42 s−1) [34,35].
Screening Natural Compounds Against the Activity of P. aeruginosa or S. pneumoniae PheRS
Natural products have been a major source of new drugs over the past 30 years and the anti-infective area in particular has been dependent on natural products and derivatives of these products [36,37]. We have screened a phyto-chemical library, a set of 320 natural products derived from plants. This approach capitalizes on recent improvements in the number and quality of natural products available. This compound library was chosen because it is rich in diverse chemotypes therefore, providing a good starting point for hit discovery. Many of the compounds are amendable to modification allowing downstream structure activity relationship (SAR) strategies. The tRNA aminoacylation assay was adapted to a scintillation proximity assay (SPA) in a 96-well microtiter plate format and used to screen the natural compounds as described in the “Methods and Materials” section. In the SPA format, this assay was very robust with a Z factor of 0.50 and a Z’ factor of 0.55 across all screening plates. The assay detects the ability of PheRS to aminoacylate tRNAPhe and in the presence of a chemical compound to measure the effect of the compound on the activity of PheRS. Chemical compounds were dissolved in 100% dimethyl sulfoxide (DMSO) resulting in final DMSO concentrations in screening assays of 4%, therefore the ability of PheRS to function in the presence of increasing amounts of DMSO was determined. There was no decrease of activity observed in aminoacylation assays for either P. aeruginosa or S. pneumoniae PheRS in assays containing up to 10% DMSO (data not shown). Initial screening assays contained chemical compounds at a concentration of 132 μM and were carried out as single point assays. Compounds observed to inhibit at least 50% of enzymatic activity were re-assayed in triplicate using the filter binding assay as described. These confirmation assays resulted in a single hit compound (BPP_03B04) resulting from the screens of both the P. aeruginosa PheRS and S. pneumoniae PheRS (Fig. 4). This compound was assayed using the tRNA aminoacylation assay in an SPA format to determine the concentration at which 50% of the enzymatic activity was inhibited (IC50) (Fig. 5). The IC50 was determined to be 2.3 μM and 4.9 μM for P. aeruginosa PheRS and S. pneumoniae PheRS, respectively.
Fig. (4).
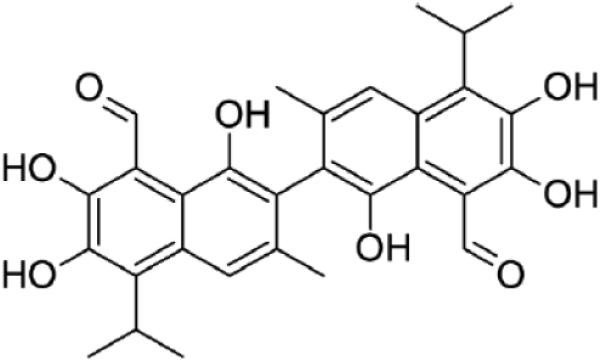
The structure of the confirmed hit compound (BPP_03B04). BPP_03B04 was identified as the sole confirmed hit compound that inhibited the activity of both P. aeruginosa and S. pneumoniae PheRS.
Fig. (5).
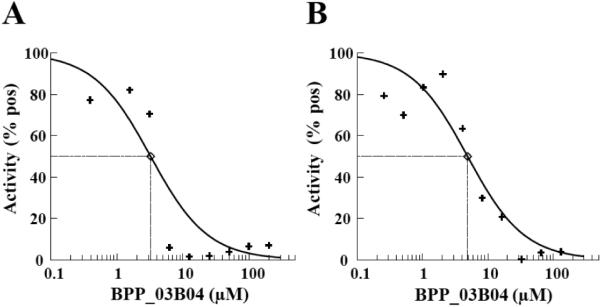
Determination of the IC50 for BPP_03B04 against the activity of P. aeruginosa and S. pneumoniae PheRS. IC50 values for the inhibitory activity of BPP_03B04 against A.) P. aeruginosa PheRS or, B.) S. pneumoniae PheRS were 2.3 μM and 4.9 μM, respectively. IC50s were determined with the test compounds serially diluted from 200 μM to 0.4 μM into aminoacylation assays containing P. aeruginosa and S. pneumoniae PheRS at 0.05 and 0.1 μM, respectively. The concentrations of ATP and Phe were held constant at 2.5 mM and 75 μM.
Wheat germ extract assays were used to determine whether BPP_03B04 inhibited protein synthesis in a eukaryotic system. Poly-U messenger RNA, yeast tRNAPhe, [3H]Phenylalanine and Mg++ concentrations were optimized for poly-Phe synthesis in wheat germ extract assays. BPP_03B04 was tested along with cycloheximide, a known inhibitor of protein synthesis [38]. In these assays protein synthesis was inhibited by cycloheximide at 30 μM [27]. In contrast, no inhibition of protein synthesis by BPP_03B04 was observed to 132 μM (Fig. 6A). PheRS from human mitochondria (hmPheRS) was previously cloned and characterized [21]. To determine if BPP_03B04 affected the activity of hmPheRS, assays containing 132 μM of the compound were carried out. In these assays, the activity of hmPheRS was observed to decrease by approximately 80% (Fig. 6B).
Fig. (6).
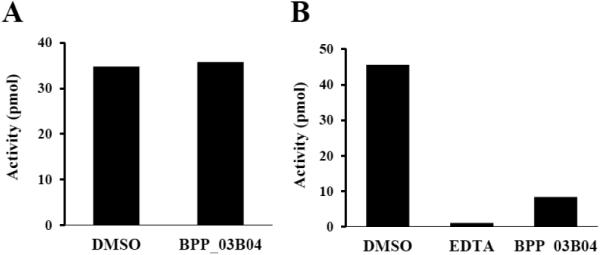
The inhibitory activity of BPP_03B04 against eukaryotic protein synthesis. A.) Inhibitory activity of BPP_03B04 in wheat germ cell cytoplasmic protein synthesis assays. B.) Inhibitory activity of BPP_03B04 against the activity of human mitochondrial PheRS. DMSO represents the positive control where only DMSO was added to the assay in the absence of the compound. EDTA represents the negative control in which reactions contained 20 mM EDTA to inhibit the activity of hmPheRS. Values are the average of three repetitive assays. The concentration of the inhibitory compound BPP_03B04 was 132 μM.
Microbiological Assays
The compound BPP_03B04 was tested in broth microdilution assays to determine minimum inhibitory concentrations (MIC) against a panel of bacteria (Table 3). The compound was tested against ten bacteria including efflux pump mutants of E. coli and P. aeruginosa and a hypersensitive strain of P. aeruginosa. BPP_03B04 was observed to inhibit E. coli and the efflux pump mutant of E. coli at concentrations of 128 μg/ml, all other organisms tested against a panel of bacteria were inhibited at concentrations of 16 or 32 μg/ml. All of the P. aeruginosa strains were inhibited with an MIC of 16 μg/ml and S. pneumoniae was inhibited with an MIC of 32 μg/ml.
Table 3.
Minimum inhibitory concentration of the compound against bacterial panel.
| Bacteria | MIC (μg/ml) |
|---|---|
| E. coli (ATCC 25922) | 128 |
| E. coli tolC | 128 |
| E. faecalis (ATCC 29212) | 32 |
| H. influenzae (ATCC 49766) | 16 |
| M. catarrhalis (ATCC 25238) | 32 |
| P. aeruginosa (ATCC 47085) | 16 |
| P. aeruginosa PAO200 (efflux mutant) | 16 |
| P. aeruginosa (hypersensitive) | 16 |
| S. aureus (ATCC 29213) | 16 |
| S. pneumoniae (ATCC 49619) | 32 |
Mechanism of Action
The IC50 for BPP_03B04 was determined using the aminoacylation assay in the SPA format at various concentrations of ATP or Phe to determine the mode of action of the inhibitor. The mechanism of inhibition of both P. aeruginosa PheRS and S. pneumoniae PheRS with respect to ATP was determined at various ATP concentrations (25, 50, 100, 250, 500, 1000 μM) ranging from 8-fold below to 5-fold above the KM. The IC50s for BPP_03B04 decreased with increasing ATP concentration (Fig. 7), which is characteristic of an uncompetitive inhibitor [39]. To determine the mechanism of inhibition with respect to the amino acid, the same assay was used, except ATP was held constant at saturating concentrations (2.5 mM) and the IC50 was determined at different concentrations of Phe (25, 50, 100, 200, 300 μM). The Phe concentrations ranged from approximately 2-fold below to 10-fold above the KM. Background amounts of free [3H]Phe in the absence of PheRS were insignificant (less than 3% of positive control). The IC50s for BPP_03B04 increased with increasing Phe concentration (Fig. 8), which is characteristic of a competitive inhibitor [39].
Fig. (7).
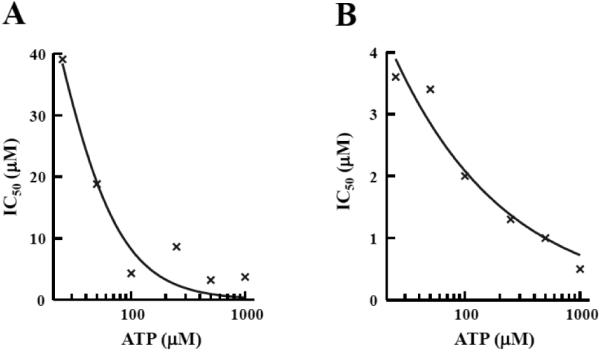
Determination of the mode of action of BPP_03B04 relative to ATP. Inhibition by BPP_03B04 is uncompetitive with ATP. BPP_03B04 IC50s were determined using the aminoacylation assay in an SPA format. The phenylalanine concentration was fixed at 100 μM, and IC50s were determined at six different ATP concentrations ranging from 25 to 1000 μM. The data was fit to the sigmoidal dose response model using XLfit (IDBS) using IC50s determined in assays containing A.) P. aeruginosa PheRS or B.) S. pneumoniae PheRS.
Fig. (8).
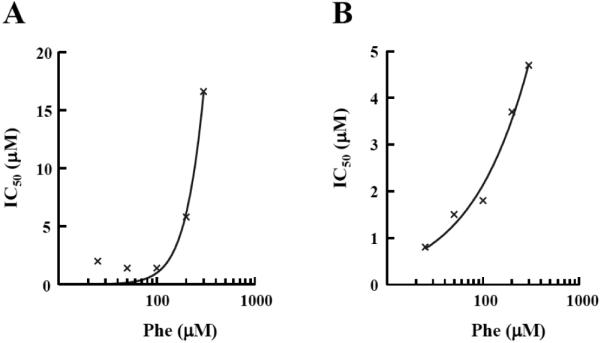
Determination of the mode of action of BPP_03B04 relative to Phe. Inhibition by BPP_03B04 is competitive with Phe. BPP_03B04 IC50s were determined using the aminoacylation assay in an SPA format. The ATP concentration was fixed at 2.5 mM, and IC50s were determined at five different Phe concentrations ranging from 25 to 300 μM. The data was fit to the sigmoidal dose response model using XLfit (IDBS) using IC50s determined in assays containing A.) P. aeruginosa PheRS or B.) S. pneumoniae PheRS.
The shift in IC50 with respect to various substrate concentrations is the product of the shift due to each individual substrate in a two-substrate reaction. For the PheRS aminoacylation assay with inhibitors that are phenylalanine competitive and ATP uncompetitive, the relationship is as follows:
This equation was used to calculate Ki for BPP_03B04 with IC50s determined at 75 μM Phe and 2.5 mM ATP. In multiple determinations, BPP_03B04 had a mean Ki of 0.83 μM with respect to P. aeruginosa PheRS and 0.98 μM with respect to S. pneumoniae PheRS.
DISCUSSION
Aminoacyl tRNA synthetases are essential proteins involved in protein synthesis in all organisms. The bacterial and cytoplasmic eukaryotic forms of the enzymes are sufficiently different as to allow the development of compounds that may inhibit the function of one form without affecting the function of the other form. However, the homolog present in mitochondria carries out the same function yet the structure may favor either the bacterial or cytoplasmic form. Functional regions of PheRS that have been shown to be conserved in T. thermophilus PheRS and others, are also conserved in PheRS from P. aeruginosa and S. pneumoniae. Previous work indicated that the human mitochondrial PheRS (hmPheRS) consists of a single polypeptide chain and is active in a monomeric form which is unlike the (αβ)2 structure of the prokaryotic and eukaryotic cytoplasmic forms of PheRS which are active in tetrameric forms. The N-terminal 314 amino acids of hmPheRS are analogous to the α-subunit of the prokaryotic form of PheRS, while the C-terminal 100 amino acids correspond to a region of the β-subunit known to interact with the anticodon stem and loop of tRNAPhe [21]. Alignments between the N-terminal 314 amino acids of hmPheRS and the corresponding amino acids in the PheRS α-subunits from P. aeruginosa and S. pneumoniae were observed to be on average 29% similar and 17% identical. Alignments of the C-terminal 100 amino acids from hmPheRS with the PheRS β-subunits from P. aeruginosa and S. pneumoniae indicated only a small level of consensus with the C-terminal ends of the prokaryotic proteins. When the α-subunits from P. aeruginosa and S. pneumoniae PheRS were compared with the human cytoplasmic homolog a similar level of conservation was observed as that seen with hmPheRS. However, when assayed in the presence of the compound BPP_03B04, activity of the wheat germ cytoplasmic form of PheRS was not affected, but the activity of the mitochondrial form of PheRS was reduced. This is similar to results seen by a group from Bayer Healthcare where the PheRS inhibitor, phenyl-thiazoleurea-solfonamide, had no effect on the cytoplasmic counterpart, but the activity of hmPheRS was significantly inhibited [40]. To determine the potential reason for this observation will require an in-depth examination of the structural aspects of the enzyme/compound interaction.
Recently, several attempts have been made to identify inhibitors of bacterial PheRS. As described above, the phenylthiazoleurea-solfonamide compounds were identified by screening E. coli PheRS against Bayer’s in-house compound library and appears to have both in vitro inhibitory activity against the enzyme and also inhibits growth of bacteria. Another compound series, ethanolamines, was identified by a group at GlaxoSmithKline using crude enzyme preparations from S. aureus [41]. These compounds were also observed to have good inhibition of enzymatic activity in vitro, however, there was no whole cell antibacterial activity. At about the same time a group from Cubist Pharmaceuticals identified series of spirocyclic furan and pyrrolidine inhibitors of PheRS that exhibited both in vitro activity as well as microbiological inhibition [42,43]. Most recently, a group from Pfizer identified a series of benzyl phenyl ethers that exhibited nano-molar IC50s, but had low levels of inhibition against cultures of wild-type Haemophilus influenza and S. pneumoniae [44]. The compounds identified in all these studies had their origins in synthetic chemistry. Throughout history, a significant role in the treatment of a wide range of medical conditions, including infectious diseases, has been played by medicinal plants. Many of the present clinically used drugs are the product of naturally occurring chemical compounds and with the rise in resistance in pathogenic bacteria, many of them are now being re-assessed as antimicrobial agents [45]. We have screened a set of natural products derived from plants to identify compounds that may inhibit the activity of PheRS from P. aeruginosa and S. pneumoniae. Many of these compounds have been previously identified as having medicinal properties. From 320 compounds, we have identified one compound that has activity against both forms of PheRS.
Compound BPP_03B04 has been identified as gossypol, a polyphenolic aldehyde extracted from cotton seed. It is thought that gossypol is produced as a natural insecticide by cotton plants [46]. Gossypol has also been studied as a male anti-fertility agent in China for a number of years [47,48]. Many of these studies have had mixed results as to the effectiveness and potential side effects of long term use [49,50]. Gossypol has also been reported to have anti-viral activity [51,52] and a certain level of anti-cancer activity has been observed [53]. Other studies showed, using a disc-diffusion method, that gossypol inhibits the growth of certain bacteria [54,55]. A compound that inhibits a number of biochemical activities may be classified as a “promiscuous” compound [56] and its usefulness as a drug candidate may be limited. To determine if this is the case with gossypol will require additional studies.
We have determined that a possible molecular target for gossypol is PheRS. When assayed against the activities of both P. aeruginosa and S. pneumoniae PheRS, gossypol inhibited the two enzymes with IC50 values in the low micromolar range. We determined that the IC50 values increased when determined at increasing concentration of the amino acid substrate indicating that gossypol was competing with Phe for binding both enzymes. Alternatively, the IC50 values decreased when determined at increasing concentration of ATP. These results indicate that PheRS inhibition by gossypol was competitive with phenylalanine binding and uncompetitive with ATP binding. It is interesting that the positive cooperativity exhibited between gossypol and ATP parallels the positive cooperativity that has been reported between phenylalanine and ATP [57,58]. The fact that increasing concentrations of ATP enhance amino acid binding fits well with the hypothesis that gossypol binds the amino acid binding site and this binding is enhanced with increasing concentrations of ATP. Thus, the naturally high physiological concentrations of ATP (2.5 mM) would serve to enhance binding of the inhibitor.
To determine if gossypol would be suitable as a compound that may be developed into an anti-bacterial agent will require more in depth studies. The level of inhibition observed when gossypol was assayed against human mitochondrial PheRS is a concern. Structure activity relationship (SAR) analysis may improve potency against bacterial protein synthesis and reduce the level of inhibition of protein synthesis in mitochondria.
ACKNOWLEDGEMENTS
The authors would like to express their gratitude to Dr. Linda Spremulli (University of North Carolina – Chapel Hill) for her critical reading of this manuscript. The authors are grateful for the financial support provided by the National Institutes of Health (grant number: 1SC3GM09817301A1). The contents of this article/publication/etc. are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. Funding was also in part from the UT Transform Grant Technology Assessment Committee.
ABBREVIATIONS
- aaRS
Aminoacyl-tRNA synthetase
- Phe
Phenylalanine
- AS
Ammonium sulfate
- DTT
Dithiothreitol
- TCA
Trichloroacetic acid
- MgOAc
Magnesium acetate
- hmPheRS
Human mitochondrial PheRS
- SPA
Scintillation proximity assay
- DMSO
Dimethyl sulfoxide
- ATP
Adenosine triphosphate
- GTP
Guanosine triphosphate
- EDTA
Ethylene diamine tetraacetic acid
- HTS
High-throughput screening
- IPTG
Isopropyl b-D-1-thiogalactopyranoside
- HEPES
4-(2-hydroxyethyl)-1-piperazine ethane sulfonic acid
- MOPS
3-(N-morpholino)propanesulfonic acid
- SAR
Structure-activity relationship
- KF
Potassium floride
- KPi
Potassium phosphate
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- [1].Maschmeyer G, Braveny I. Review of the incidence and prognosis of Pseudomonas aeruginosa infections in cancer patients in the 1990s. Eur J Clin Microbiol Infect Dis. 2000;19(12):915–25. doi: 10.1007/s100960000410. [DOI] [PubMed] [Google Scholar]
- [2].Giamarellou H, Antoniadou A. Antipseudomonal antibiotics. Med Clin North Am. 2001;85:19–42. doi: 10.1016/s0025-7125(05)70303-5. [DOI] [PubMed] [Google Scholar]
- [3].Roussel P, Lamblin G. The glycosylation of airway mucins in cystic fibrosis and its relationship with lung infection by Pseudomonas aeruginosa. Adv Exp Med Biol. 2003;535:17–32. doi: 10.1007/978-1-4615-0065-0_2. [DOI] [PubMed] [Google Scholar]
- [4].Oliver A, Canton R, Campo P, Baquero F, Blazquez J. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science. 2000;288:1251–4. doi: 10.1126/science.288.5469.1251. [DOI] [PubMed] [Google Scholar]
- [5].Sahm DF, Draghi DC, Master RN, et al. Pseudomonas aeruginosa antimicrobial resistance update: US resistance trends from 1998 to 2001; 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC).2002. [Google Scholar]
- [6].Nouer SA, Pinto M, Teixeira L, Nucci M. Risk Factors for multidrug resistant Pseudomonas aeruginosa (MDRPa) colonization or infection in hospitalized patients; 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC).2002. [Google Scholar]
- [7].Center for Disease Control and Prevention: Pneumococcal Disease 2014 Available at: http://www.cdc.gov/pneumococcal/about/index.html. Last accessed July 21, 2014.
- [8].Henriques-Normark B, Tuomanen EI. The pneumococcus: epidemiology, microbiology, and pathogenesis. Cold Spring Harb Perspect Med. 2013;3(7) doi: 10.1101/cshperspect.a010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Klugman KP. Pneumococcal resistance to antibiotics. Clin Microbiol Rev. 1990;3:171–96. doi: 10.1128/cmr.3.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Van BF, Reinert RR, Appelbaum PC, Tulkens PM, Peetermans WE. Multidrug-resistant Streptococcus pneumoniae infections: current and future therapeutic options. Drugs. 2007;67:2355–82. doi: 10.2165/00003495-200767160-00005. [DOI] [PubMed] [Google Scholar]
- [11].Farrell DJ, Castanheira M, Mendes RE, Sader HS, Jones RN. In vitro activity of ceftaroline against multidrug-resistant Staphylococcus aureus and Streptococcus pneumoniae: a review of published studies and the AWARE Surveillance Program (2008-2010) Clin Infect Dis. 2012;(Suppl 3):S206–S214. doi: 10.1093/cid/cis563. [DOI] [PubMed] [Google Scholar]
- [12].Flamm RK, Sader HS, Farrell DJ, Jones RN. Summary of ceftaroline activity against pathogens in the United States, 2010: report from the Assessing Worldwide Antimicrobial Resistance Evaluation (AWARE) surveillance program. Antimicrob Agents Chemother. 2012;56:2933–40. doi: 10.1128/AAC.00330-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Eriani G, Delarue M, Poch O, Gangloff J, Moras D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature. 1990;347:203–6. doi: 10.1038/347203a0. [DOI] [PubMed] [Google Scholar]
- [14].Moras D. Structural and functional relationships between aminoacyl-tRNA synthetases. Trends Biochem Sci. 1992;17:159–64. doi: 10.1016/0968-0004(92)90326-5. [DOI] [PubMed] [Google Scholar]
- [15].Cusack S. Eleven down and nine to go. Nat Struct Biol. 1995;2:824–31. doi: 10.1038/nsb1095-824. [DOI] [PubMed] [Google Scholar]
- [16].Francklyn C, Musier-Forsyth K, Martinis SA. Aminoacyl-tRNA synthetases in biology and disease: new evidence for structural and functional diversity in an ancient family of enzymes. RNA. 1997;3:954–60. [PMC free article] [PubMed] [Google Scholar]
- [17].Baltzinger M, Fasiolo F, Remy P. Yeast phenylalanyl-tRNA synthetase. Affinity and photoaffinity labelling of the stereospecific binding sites. Eur J Biochem. 1979;97:481–94. doi: 10.1111/j.1432-1033.1979.tb13136.x. [DOI] [PubMed] [Google Scholar]
- [18].Goldgur Y, Mosyak L, Reshetnikova L, et al. The crystal structure of phenylalanyl-tRNA synthetase from Thermus thermophilus complexed with cognate tRNAPhe. Structure. 1997;5:59–68. doi: 10.1016/s0969-2126(97)00166-4. [DOI] [PubMed] [Google Scholar]
- [19].Mosyak L, Reshetnikova L, Goldgur Y, Delarue M, Safro MG. Structure of phenylalanyl-tRNA synthetase from Thermus thermophilus. Nat Struct Biol. 1995;2(7):537–47. doi: 10.1038/nsb0795-537. [DOI] [PubMed] [Google Scholar]
- [20].Schimmel P, Tao J, Hill J. Aminoacyl tRNA synthetases as targets for new anti-infectives. FASEB J. 1998;12(15):1599–1609. [PubMed] [Google Scholar]
- [21].Bullard JM, Cai YC, Demeler B, Spremulli LL. Expression and characterization of a human mitochondrial phenylalanyl-tRNA synthetase. J Mol Biol. 1999;288(4):567–77. doi: 10.1006/jmbi.1999.2708. [DOI] [PubMed] [Google Scholar]
- [22].Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- [23].Cull MG, McHenry CS. Purification of Escherichia coli DNA polymerase III holoenzyme. Methods Enzymol. 1995;262:22–35. doi: 10.1016/0076-6879(95)62005-2. [DOI] [PubMed] [Google Scholar]
- [24].Martinis SA, Fox GE. Non-standard amino acid recognition by Escherichia coli leucyl-tRNA synthetase. Nucl Acids Symp Ser. 1997;36:125–8. [PubMed] [Google Scholar]
- [25].Macarron R, Mensah L, Cid C, et al. A homogeneous method to measure aminoacyl-tRNA synthetase aminoacylation activity using scintillation proximity assay technology. Anal Biochem. 2000;284(2):183–90. doi: 10.1006/abio.2000.4665. [DOI] [PubMed] [Google Scholar]
- [26].Methods for Dilution Antimicrobial Susceptibility Test for Bacteria that Grow Aerobically. 2. Vol. 23. Clinical and laboratory Standards Institute (formerly NCCLS); 2006. M7-A7. [Google Scholar]
- [27].Ribble W, Hill WE, Ochsner UA, et al. Discovery and analysis of 4H-pyridopyrimidines, a class of selective bacterial protein synthesis inhibitors. Antimicrob Agents Chemother. 2010;54(11):4648–57. doi: 10.1128/AAC.00638-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Reshetnikova L, Moor N, Lavrik O, Vassylyev DG. Crystal structures of phenylalanyl-tRNA synthetase complexed with phenylalanine and a phenylalanyl-adenylate analogue. J Mol Biol. 1999;287(3):555–68. doi: 10.1006/jmbi.1999.2617. [DOI] [PubMed] [Google Scholar]
- [29].Moor N, Kotik-Kogan O, Tworowski D, Sukhanova M, Safro M. The crystal structure of the ternary complex of phenylalanyl-tRNA synthetase with tRNAPhe and a phenylalanyl-adenylate analogue reveals a conformational switch of the CCA end. Biochemistry. 2006;45(35):10572–83. doi: 10.1021/bi060491l. [DOI] [PubMed] [Google Scholar]
- [30].Fishman R, Ankilova V, Moor N, Safro M. Structure at 2.6 A resolution of phenylalanyl-tRNA synthetase complexed with phenylalanyl-adenylate in the presence of manganese. Acta Crystallogr D Biol Crystallogr. 2001;57:1534–44. doi: 10.1107/s090744490101321x. Pt 11. [DOI] [PubMed] [Google Scholar]
- [31].Mermershtain I, Finarov I, Klipcan L, Kessler N, Rozenberg H, Safro MG. Idiosyncrasy and identity in the prokaryotic Phe-system: crystal structure of E. coli phenylalanyl-tRNA synthetase complexed with phenylalanine and AMP. Protein Sci. 2011;20(1):160–7. doi: 10.1002/pro.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Evdokimov AG, Mekel M, Hutchings K, et al. Rational protein engineering in action: the first crystal structure of a phenylalanine tRNA synthetase from Staphylococcus haemolyticus. J Struct Biol. 2008;162(1):152–69. doi: 10.1016/j.jsb.2007.11.002. [DOI] [PubMed] [Google Scholar]
- [33].Abibi A, Ferguson AD, Fleming PR, Gao N, Hajec LI, Hu J, et al. The role of a novel auxiliary pocket in bacterial phenylalanyl-tRNA synthetase druggability. J Biol Chem. 2014;289(31):21651–62. doi: 10.1074/jbc.M114.574061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Peterson ET, Uhlenbeck OC. Determination of recognition nucleotides for Escherichia coli phenylalanyl-tRNA synthetase. Biochemistry. 1992;31(42):10380–89. doi: 10.1021/bi00157a028. [DOI] [PubMed] [Google Scholar]
- [35].Ibba M, Kast P, Hennecke H. Substrate specificity is determined by amino acid binding pocket size in Escherichia coli phenylalanyl-tRNA synthetase. Biochemistry. 1994;33(23):7107–12. doi: 10.1021/bi00189a013. [DOI] [PubMed] [Google Scholar]
- [36].Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75(3):311–35. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Butler MS, Buss AD. Natural products--the future scaffolds for novel antibiotics? Biochem Pharmacol. 2006;71(7):919–29. doi: 10.1016/j.bcp.2005.10.012. [DOI] [PubMed] [Google Scholar]
- [38].Satav JG, Katyare SS, Fatterparker P, Sreenivasan A. Study of protein synthesis in rat liver mitochondria use of cycloheximide. Eur J Biochem. 1997;73(1):287–96. doi: 10.1111/j.1432-1033.1977.tb11318.x. [DOI] [PubMed] [Google Scholar]
- [39].Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22(23):3099–108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- [40].Beyer D, Kroll HP, Endermann R, et al. New class of bacterial phenylalanyl-tRNA synthetase inhibitors with high potency and broad-spectrum activity. Antimicrob Agents Chemother. 2004;48(2):525–32. doi: 10.1128/AAC.48.2.525-532.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jarvest RL, Erskine SG, Forrest AK, et al. Discovery and optimisation of potent, selective, ethanolamine inhibitors of bacterial phenylalanyl tRNA synthetase. Bioorg Med Chem Lett. 2005;15(9):2305–09. doi: 10.1016/j.bmcl.2005.03.003. [DOI] [PubMed] [Google Scholar]
- [42].Yu XY, Finn J, Hill JM, et al. A series of heterocyclic inhibitors of phenylalanyl-tRNA synthetases with antibacterial activity. Bioorg Med Chem Lett. 2004;14(5):1343–6. doi: 10.1016/j.bmcl.2003.11.082. [DOI] [PubMed] [Google Scholar]
- [43].Yu XY, Finn J, Hill JM, et al. A series of spirocyclic analogues as potent inhibitors of bacterial phenylalanyl-tRNA synthetases. Bioorg Med Chem Lett. 2004;14(5):1339–42. doi: 10.1016/j.bmcl.2003.11.081. [DOI] [PubMed] [Google Scholar]
- [44].Montgomery JI, Toogood PL, Hutchings KM, et al. Discovery and SAR of benzyl phenyl ethers as inhibitors of bacterial phenylalanyl-tRNA synthetase. Bioorg Med Chem Lett. 2009;19(3):665–69. doi: 10.1016/j.bmcl.2008.12.054. [DOI] [PubMed] [Google Scholar]
- [45].Mahady GB, Huang Y, Doyle BJ, Locklear T. Natural products as antibacterial agents. In: Atta UR, editor. Studies in Natural Products Chemistry Bioactive Natural Products (Part O) 35 Elsevier; 2008. pp. 423–44. [Google Scholar]
- [46].Sharma HC, Agarwal RA. Effect of some antibiotic compounds in gossypium on the post-embryonic development of spotted bollworm (Earias vittella) Entomologia Experimentalis et Applicata. 1982;31(2-3):225–28. [Google Scholar]
- [47].Liu GZ, Lyle KC, Cao J. Clinical trial of gossypol as a male contraceptive drug. Part I. Efficacy study. Fertil Steril. 1987;48(3):459–61. doi: 10.1016/s0015-0282(16)59418-7. [DOI] [PubMed] [Google Scholar]
- [48].Meng GD, Zhu JC, Chen ZW, et al. Follow-up of men in the recovery period immediately after the cessation of gossypol treatment. Contraception. 1988;37(2):119–128. doi: 10.1016/0010-7824(88)90122-9. [DOI] [PubMed] [Google Scholar]
- [49].Gu ZP, Mao BY, Wang YX, et al. Low dose gossypol for male contraception. Asian J Androl. 2000;2(4):283–287. [PubMed] [Google Scholar]
- [50].Waites GM, Wang C, Griffin PD. Gossypol: reasons for its failure to be accepted as a safe, reversible male antifertility drug. Int J Androl. 1998;21(1):8–12. doi: 10.1046/j.1365-2605.1998.00092.x. [DOI] [PubMed] [Google Scholar]
- [51].Radloff RJ, Deck LM, Royer RE, Vander Jagt DL. Antiviral activities of gossypol and its derivatives against herpes simplex virus type II. Pharmacol Res Commun. 1986;18(11):1063–73. doi: 10.1016/0031-6989(86)90023-8. [DOI] [PubMed] [Google Scholar]
- [52].Polsky B, Segal SJ, Baron PA, Gold JW, Ueno H, Armstrong D. Inactivation of human immunodeficiency virus in vitro by gossypol. Contraception. 1989;39(6):579–87. doi: 10.1016/0010-7824(89)90034-6. [DOI] [PubMed] [Google Scholar]
- [53].Oliver CL, Miranda MB, Shangary S, Land S, Wang S, Johnson DE. (−)-Gossypol acts directly on the mitochondria to overcome Bcl-2- and Bcl-X(L)-mediated apoptosis resistance. Mol Cancer Ther. 2005;4(1):23–31. [PubMed] [Google Scholar]
- [54].Margalith P. Inhibitory effect of gossypol on microorganisms. Appl Microbiol. 1967;15(4):952–3. doi: 10.1128/am.15.4.952-953.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Przybylski P, Pyta K, Stefanska J, et al. Synthesis, crystal structures and antibacterial activity studies of aza-derivatives of phytoalexin from cotton plant--gossypol. Eur J Med Chem. 2009;44(11):4393–4403. doi: 10.1016/j.ejmech.2009.05.032. [DOI] [PubMed] [Google Scholar]
- [56].Baell JB. Observations on screening-based research and some concerning trends in the literature. Future Med Chem. 2010;2(10):1529–46. doi: 10.4155/fmc.10.237. [DOI] [PubMed] [Google Scholar]
- [57].Kosakowski HM, Holler E. Phenylalanyl-tRNA synthetase from Escherichia coli K10. Synergistic coupling between the sites for binding of L-phenylalanine and ATP. Eur J Biochem. 1973;38(2):274–82. doi: 10.1111/j.1432-1033.1973.tb03059.x. [DOI] [PubMed] [Google Scholar]
- [58].Pimmer J, Holler E. Mechanism of phenylalanyl-tRNA synthetase of Escherichia coli K10. Modulation of catalytic properties by magnesium. Biochemistry. 1979;18(17):3714–23. doi: 10.1021/bi00584a012. [DOI] [PubMed] [Google Scholar]