Abstract
Objective(s):
In the present study, we evaluated immunological and immunomodulatory properties of royal jelly (RJ) in 2,4,6 trinitrobenzene sulfonic acid (TNBS)-induced colitis in rats.
Materials and Methods:
Eighteen adult female Wistar albino rats were divided into three groups of six animals each: a control group that received only saline solution, a TNBS-induced colitis group, and a TNBS-colitis+RJ group that received 250 mg/kg/day of RJ for seven days before the induction of colitis, following by the same treatment for an additional seven days. At the end of the experiment, cardiac blood and colon samples were obtained under deep anaesthesia from the animals in all groups. Serum interleukin-1β (IL-1β), tumour necrosis factor-alpha (TNF-α) and IL-10 levels were analyzed with an enzyme-linked immunosorbent assay (ELISA). Five-micrometre-thick sections were stained with haematoxylin-eosin (H&E) for microscopic evaluations. For immunohistochemical evaluations, the paraffin sections were stained with anti-CD3 (cluster of differentiation), anti-CD5, anti-CD8 and anti-CD45.
Results:
The results showed that the oral RJ treatment inhibited proinflammatory cytokines, IL-1β and TNF-α secretion, while increasing anti-inflammatory cytokine IL-10 production in the TNBS-induced colitis+RJ group compared with the colitis group not treated with RJ. The colitis was not as severe in the colitis+RJ group, with ulcerative damage, weight loss and inflammatory scores suggesting that impaired CD3-, CD5-, CD8- and CD45-positive T cell immune responses likely mediated the anti-inflammatory effect.
Conclusion:
The antioxidant and anti-inflammatory properties of RJ protected colon mucosa against TNBS-induced colitis in rats orally treated with RJ.
Keywords: Colitis, Rats, Royal jelly, TNBS
Introduction
Ulcerative colitis and Crohn’s disease are chronic inflammatory bowel diseases (IBDs). IBD refers to a chronic noninfectious inflammation of unknown etiology targeting one or more sites of the gastrointestinal tract (1). Epidemiological studies suggest that the prevalence of IBDs has increased since 1950, but these rations are set to stabilize in Western Europe and North America, it has an increasing trend in South America, Asia and Pacific regions (2).
Chronic inflammation in IBD is thought to be the result of irregularity between inflammatory cytokines, such as interleukin-1 beta (IL1-β), IL-10, tumor necrosis factor-alpha (TNF-α) and transforming growth factor-beta (TGF-β) (2). TNF-α seems to play a major role in the duration of inflammation in Crohn’s disease. One study reported that levels of TNF-α were increased in the blood, as well as in the feces, of patients with active Crohn’s disease (3).
In addition to the aforementioned changes in patients with IBD, genetic and environmental factors are thought to play a major role in the disease (4). In one study, CD4-positive lymphocytes with a type 1 helper T-cell phenotype dominated the mucosa of patients with established Crohn’s disease (5).
There are several herbal products, including honey, with suggested antioxidant, anti-inflammatory, antiulcerative and antipyretic properties (6). Royal jelly (RJ) is a secretion of the mandibular and hypo-pharyngeal glands of worker honeybees. Laboratory studies have suggested that it exhibits antihyper-glycemic (7), antioxidant (8),anti-inflammatory (9) and immunomodulatory (10, 11) properties. In addition, one study demonstrated a protective effect of RJ against acetic acid-induced colitis in rats (12). The previous study suggested that Teucrium polium had effective protection against acetic acid induced ulcerative colitis in the dog as an animal model (13). Furthermore, Tanideh et al (2014) showed that strawberry extracts in different doses therapy could restore the body weight and result in a healing effect in acetic acid-induced colonic tissue damage (14).
The goal of this study was to evaluate the protective, antiulcerative and immunomodulatory effects of RJ in 2,4,6 trinitrobenzene sulfonic acid (TNBS)-induced colitis by determining changes in serum levels of IL-1β, TNF- α and IL-10, and changes in the distribution of T-lymphocytes.
Materials and Methods
Animals
Eighteen female Wistar albino rats weighing 220 to 275 g were obtained from the Experimental Animal Centre of Trakya University (Edirne, Turkey). The animals were housed under specific pathogen-free conditions and kept in optimum laboratory conditions (temperature: 22±2 °C; humidity: 50–55%; light/dark period: 12 hr/12 hr). All animals were fed a standard laboratory diet and had access to tap water ad libitum. All experimental procedures described in this study were performed in accordance with the guidelines of the local ethics committee for animal studies (TUHDYEK-2012/40).
Induction of colitis
All the rats were fasted overnight before the induction of colitis. After the animals were lightly anesthetized with xylazine (Rompun, Bayer, Istanbul, Turkey) and ketamine (Pfizer, Istanbul, Turkey) intraperitoneally (IP), a rubber catheter was inserted rectally into the colon, with the tip 8 cm proximal to the anus. TNBS (25 mg/rat; Sigma, MA, USA) was dissolved in 50% (v/v) ethanol (total volume, 0.8 ml) and then injected slowly into the lumen of the colon as previously described (15). Thereafter, the animals were maintained for 45 sec in a Trendelenburg position to avoid reflux. The animals in the control group received 0.8 ml of normal saline solution in the same way.
Experimental design
Eighteen female Wistar albino rats were randomly divided into three groups: a control group (n=6) that received only saline solution, a colitis group (n=6) that received TNBS (intracolonic) and a colitis+RJ group (n=6) that received TNBS (intracolonic) and RJ (250 mg/kg) daily via an intragastric tube. The RJ was purchased from a local natural food store (Istanbul, Turkey). The colitis+RJ group received the RJ for seven days before the induction of colitis, followed by treatment with the RJ for an additional seven days.
Collection of tissue samples
The rats were anesthetized with xylazine (10 mg/kg/bw) and ketamine (90 mg/kg/bw) IP, and sacrificed by cervical dislocation 24 hr after the last treatment. The abdomen was opened by a midline incision. The distal colon was rapidly excised and opened along the antimesenteric border. The fecal contents were removed, and the colon was rinsed with 0.9% saline for macroscopic scoring and histological and immunohistochemical examination. Intracardiac blood samples were collected for cytokine measurement. The samples were centrifuged, and the sera were stored at -80 °C until analysis. In addition, changes in the animals’ body weights were recorded before and after the induction of colitis.
Evaluation of macroscopic colonic damage
Colon damage (macroscopic damage score) was evaluated and scored by two independent observers as described previously (16) (Table 1).
Table 1.
Criteria for macroscopic scoring of colonic damage
| Feature | Score |
|---|---|
| Ulceration | |
| Normal appearance | 0 |
| Focal hyperemia, no ulcers | 1 |
| Ulceration without hyperemia or bowel wall thickening | 2 |
| Ulceration with inflammation at one site | 3 |
| Two or more sites with ulceration and inflammation | 4 |
| Major sites of damage extending >1 cm along the length of the colon | 5 |
| When an area of damage extended >2 cm along length of colon, the score of was increased by 1 for each additional cm of involvement | 6–10 |
| plus | |
| Adhesions | |
| No adhesions | 0 |
| Minor adhesions (colon can be easily separated from other tissue) | 1 |
| Major adhesions | 2 |
| Diarrhea | |
| No | 0 |
| Yes | 1 |
| Thickness | |
| Maximal bowel wall thickness (x), in mm, was added to above score x | x |
| Total score |
Evaluation of microscopic colonic damage
The distal colon samples were fixed in 10% neutral buffered formalin solution for 24 hr and embedded in paraffin. Serial 5 µm sections were cut and stained with haematoxylin-eosin (H&E). The colonic histological changes (microscopic damage score) were evaluated and scored by two independent observers as follows (17): epithelium (E): 0, normal morphology; 1, loss of goblet cells; 2, loss of goblet cells in large areas; 3, loss of crypts; 4, loss of crypts in large areas. Infiltration (I): 0, no infiltrate; 1, infiltrate around crypt basis; 2, infiltrate reaching to lamina muscularis mucosae; 3, extensive infiltration reaching the L. muscularis mucosae and thickening of the mucosa with abundant edema; 4, infiltration of the L. submucosa. The total histological score represents the sum of the epithelium and infiltration score (total score= E+I).
Immunohistochemistry
Sections were deparaffinized in xylene and rehydrated in a graded series of ethanol and then boiled in citrate buffer (10 mM; pH 6.0, Thermo Scientific/Lab Vision, Fremont, CA, USA) for 10 min for antigen retrieval. Following washing with phosphate buffer saline (PBS), the sections were immersed in 3% H2O2 (in distilled water) for 10 min to inhibit endogenous peroxidase activity. The nonspecific binding of antibodies was blocked by incubation with a blocking serum (Thermo Scientific/Lab Vision) at room temperature for 5 min. The sections were incubated with rabbit polyclonal primary antibodies (anti-CD3, anti-CD5, anti-CD8 and anti-CD45) (Abbiotec, San Diego, CA, USA, dilution 1/100) at room temperature for 60 min. They were then washed three times with PBS and incubated with biotinylated secondary antibody (Ultra Vision Detection System-HRP kit, Thermo Scientific/Lab Vision). Streptavidin peroxidase (Ultra Vision Detection System-HRP kit, Thermo Scientific/Lab Vision) was then added at room temperature for 10 min. The chromogen 3-amino-9-ethyl-carbazole (AEC Substrate System, Thermo Scientific/Lab Vision) was used, and the sections were counter-stained with haematoxylin.
The tissue sections were examined under light microscopy (×400), and numbers of CD-positive cells per square millimeter were counted in random high-power fields using an Olympus BX51 light microscope (Tokyo, Japan) incorporating a square graticule in the eyepiece (eyepiece ×10, objective ×40). The CD-positive cells in the colon preparations of each group were counted in 100 high-power fields. The number of positive cells/mm2 was recorded.
Measurement of cytokine levels
The blood samples were centrifuged at 10,000×g for 10 min. The sera were stored at -80 °C until assayed. Levels of IL-1β, TNF-α and IL-10 were analyzed with murine enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. All samples were assayed in duplicate.
Statistical analysis
All statistical analyses were completed using SPSS statistical software (SPSS for Windows, version 12.0). A one-way analysis of variance and Tukey’s post-test were used to compare the control and experimental groups. A value of P<0.05 was considered statistically significant. All values were expressed as mean ±standard deviation.
Results
The control animals showed significant changes in body weight compared with the colitis and colitis+RJ groups (P<0.05). However, the body weight loss in the colitis+RJ group was less than in the colitis group not treated with RJ (P<0.05) (Figure 1A). The colon weight/length ratio was significantly higher in both colitis-induced groups (Figure 1B).
Figure 1.
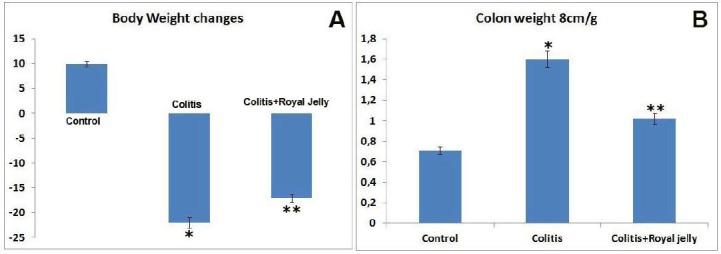
Body weight changes (A) and colon tissue weights (B). * P<0.05 compared with the control group; ** P<0.05 compared with the control and colitis groups
Macroscopic examination of the colon in the colitis groups showed serious colonic mucosal ulceration, edema and hemorrhage when compared with the control group (Figure 2A and B). In addition, the macroscopic colitis score of the TNBS-induced colitis group was significantly higher than that of the control group (Figure 2D). In contrast, the macroscopic score of the colitis+RJ group was significantly decreased compared to that of the colitis group not treated with RJ (Figure 2C and D).
Figure 2.
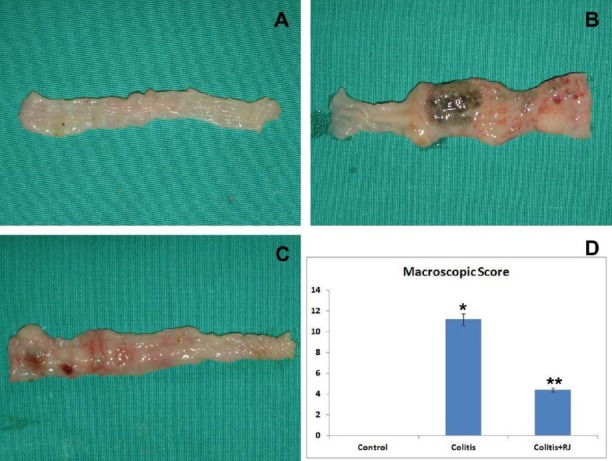
Macroscopic appearance and macroscopic damage score. Control (A); TNBS-induced colitis (B); TNBS-induced rats that received the RJ treatment (C); Macroscopic damage score (D). * P<0.05 compared with the control group; ** P<0.05 compared with the control and colitis groups
The microscopic images of the colon sections of the control group were normal (Figure 3A). In the histological examinations, TNBS-induced colitis was characterized by mucosal ulceration and severe leukocyte infiltration in the mucosa and submucosa, in addition to reduced infiltration in the muscular layers. The colitis group had a significantly higher microscopic score compared to the control group (Figure 3B and D; P<0.05). However, the microscopic score of the colitis+RJ group was significantly decreased compared with the colitis group not treated with RJ (Figure 3C and D; P<0.05).
Figure 3.
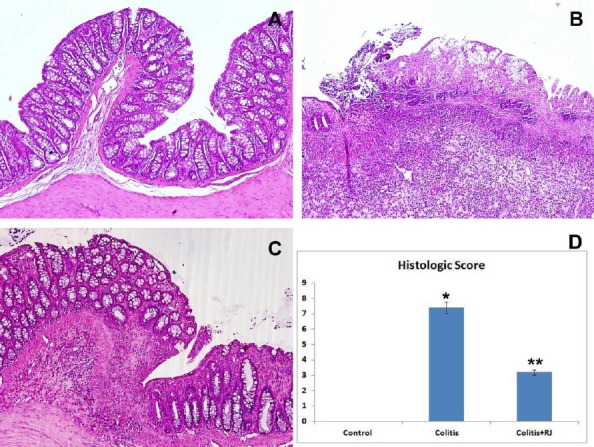
Histological appearance of colon tissue. Control (A), showing no histological changes in the colon samples of the control rats; TNBS-induced colitis (B), showing edema, ulceration and strong inflammation of the mucosa reaching the submucosal layer of the colon; colitis+RJ (C), showing slight ulceration and inflammatory infiltration. (H&E; all magnifications: 100×); histological score (D). * P<0.05 compared with the control group; ** P<0.05 compared with the control and colitis groups
Immunohistochemical evaluation
The numbers of mucosal and submucosal CD3-, CD5-, CD8- and CD45-positive T cells were significantly different among the control and experimental groups. Although they were highest in both colitis groups, they were decreased significantly in the RJ+colitis group. The histological appearance and distribution of immunopositive T cells are summarized in Figure 4–7. In all groups, CD3-, CD5-, CD8- and CD45-positive T cells were mainly observed in the subepithelial region, between the crypts and lamina propria. They were rarely observed in the lamina epithelialis.
Figure 4.
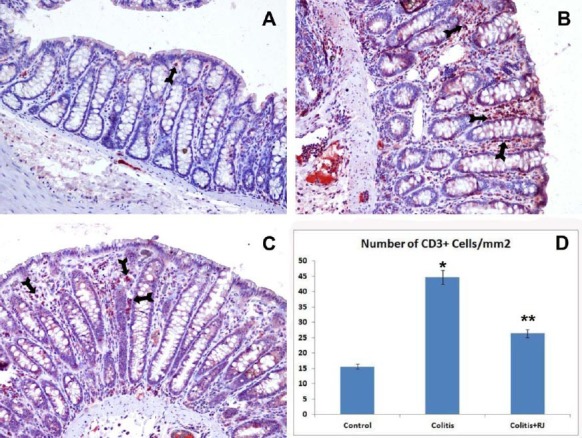
CD3-positive T lymphocytes. Control (A); TNBS-induced colitis (B); colitis+ RJ (C) (immunoperoxidase, haematoxylin counterstain, 200×); Distribution and number of CD3-positive T cells (Arrows) (D). * P<0.05 compared with the control group; ** P<0.05 compared with the control and colitis groups
Figure 5.
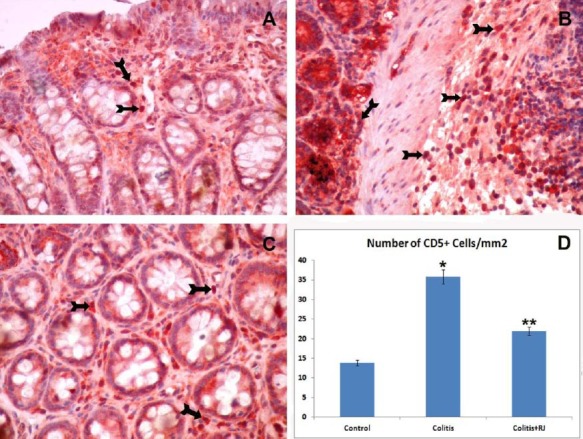
CD5-positive T lymphocytes. Control (A); TNBS-induced colitis (B); colitis+RJ (C) (immunoperoxidase, haematoxylin counterstain, 400×); Distribution and number of CD5-positive T cells (Arrows) (D). * P<0.05 compared with the control group; ** P<0.05 compared with the control and colitis groups
Figure 6.
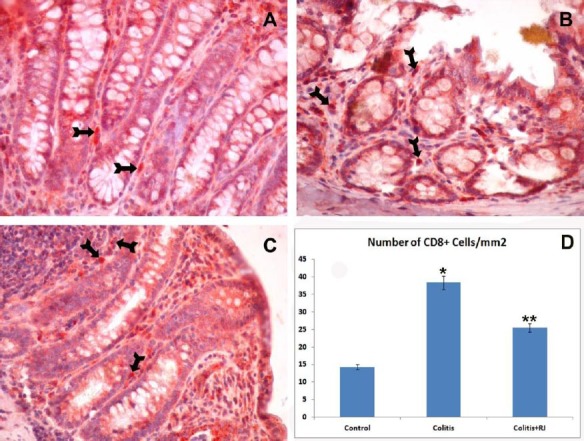
CD8-positive T lymphocytes. Control (A); TNBS-induced colitis (B); colitis+RJ (C) (immunoperoxidase, haematoxylin counterstain, 400×); Distribution of number of CD8-positive T cells (Arrows) (D). * P<0.05 compared with the control group; ** P<0.05 compared with the control and colitis groups
Figure 7.
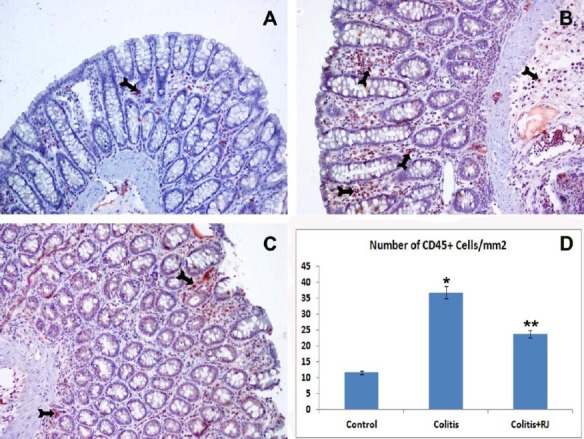
CD45-positive T lymphocytes. Control (A); TNBS-induced colitis (B); colitis+RJ (C) (immunoperoxidase, haematoxylin counterstain, 200×); Distribution and number of CD45 positive T cells (Arrows) (D). * P<0.05 compared with the control group; ** P<0.05; compared with the control and colitis groups
Biochemical evaluation
The levels of TNF-α and IL-1β in the serum of the colitis groups were significantly increased compared to the control group (P<0.05), but they were markedly lower in the RJ+colitis group compared with the colitis group not treated with RJ (P<0.05). The serum levels of IL-10 were significantly lower in both colitis groups compared to the control group. However, they were significantly higher in the RJ+colitis group than in the colitis group not treated with RJ (Figure 8).
Figure 8.
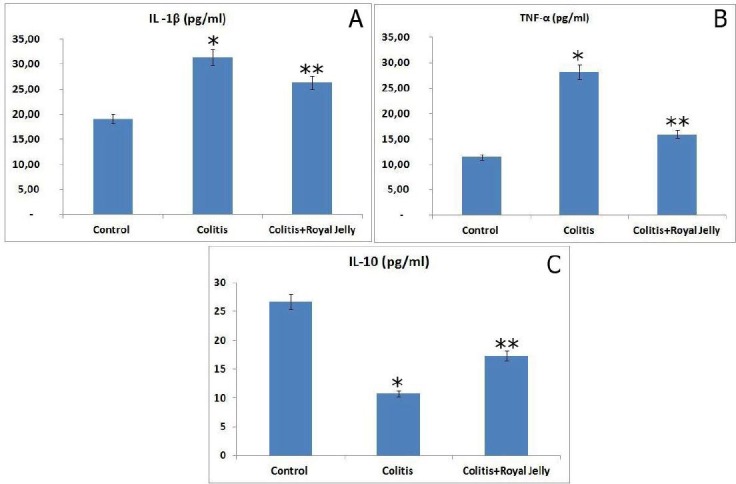
Serum cytokine levels. IL-1β (A), TNF-α (B) and IL-10 (C). * P<0.05 compared with the control group; ** P<0.05 compared with the control and colitis groups
Discussion
According to previous research, the TNBS model of experimental colitis is suitable for screening drugs with anticolitic activity, and many of its pathological and clinical properties are similar to those of human ulcerative colitis (18). In the present study, we assessed the effect of RJ on the immunopathogenesis of ulcerative colitis to determine whether it can down-regulate the inflammatory immune response during ulcerative colitis.
Previous studies of an experimental model of ulcerative colitis, which is characterized by an acute inflammatory response, observed thickening of the colon mucosa and submucosa, ulcerations and immune cell infiltration (19,20). In the present study, TNBS-induced experimental colitis was indicated by the presence of macroscopic and microscopic lesions corresponding to an increase in the colonic weight. We found that it was accompanied by significant edema, injuries to the mucosa, focal ulcerations and increased polymorphonuclear leukocyte inflamma-tions in the colon mucosa and submucosa.
Bilsel et al showed that natural honey had protective effects in acetic acid-induced and TNBS-induced IBD models (21). Similarly, Prakash et al suggested that honey is effective in treating IBD (6). Previous studies suggested that RJ has anti-inflammatory, DNA-protective and antitumor effects in experimental animals (9, 22). According to one animal study, the anticolitogenic effects of RJ might be due to it increasing the mucin content of the colon mucosa, thereby improving the animal’s antioxidant status (10).
In the present study, RJ (250 mg/kg/day) was effective in treating specific mucosal elements of TNBS-induced colitis. It improved epithelial healing and mucosal thickness, returning both to normal values in the colon. Mehrabani et al (2011) showed that Calendula officinalis had protective effects in acetic acid-induced and TNBS-induced IBD models: a significant mucosal recovery after administration of 30 and 45 days (23).
Serum levels of IL-1β, IL-6, IL-10, IL-17, IL-23 and TNF-α play a key role in the pathogenesis of IBD (24,25). Fazio et al showed that chronic dextran sodium sulphate (DSS)-induced colitis in mice significantly increased serum levels of IL-1β, IL-6, IL-10, TNF-α and IL-17 (26). In another DSS-induced colitis model, Celinski et al suggested that colitis increased serum and intestinal homogenate levels of cytokines IL-2, IL-4 and IL-10 (27, 28). In the present study of TNBS-induced colitis, the serum IL-1β and TNF-α levels increased significantly. The activation of IL-1β and TNF-α is associated with the formation of inflammatory colitis. IL-1β and TNF-α levels were decreased in the RJ+colitis group. The decrease may be due to RJ regulating free-radical scavenging activity, particularly reactive oxygen species, and the release of proinflammatory cytokines (29). In our study, serum levels of IL-1β and TNF-α were highest in the colitis groups. However, the serum levels of IL-10 were lower in the colitis group not treated with RJ than in the control and RJ+colitis groups. Similarly, Peng et al and Wang et al suggested that serum levels of IL-10 decreased in ulcerative colitic rat models (30, 31).
In our model of colitis, we found that the infiltration of CD3-, CD4-, CD8- and CD45-positive cells increased after TNBS administration in both groups, but it declined at the end of the healing process in the colitis+RJ group. These findings suggest that RJ prevented the infiltration of lymphocytes. Furthermore, in a previous study, we demonstrated that numbers of intestinal lymphocytes increased during inflammatory bowel diseases (10).
Previous studies suggested that RJ has wound-healing (9, 10, 32), immunomodulatory and antiallergic properties in experimental animals (33). Vucevic et al proposed that the high concentration of RJ in fatty acids inhibited the proliferation of allogeneic T cells (34). In the same study, lower concentrations of fatty acids inhibited the maturation of dendritic cells, and RJ fatty acids up-regulated the production of IL-10 and down-regulated the production of IL-12. Furthermore, Karaca et al suggested that RJ treatment decreased the number of CD3-, CD5-, CD45- and CD68-positive cells in acetic acid-induced colitis rats (10).
In the present study, histologically, as evidenced by the reduction in inflammatory infiltrates, the RJ treatment resulted in recovery from the inflammatory process. Biochemically, it ameliorated serum cytokine levels.
Karaca et al found a potential protective effect of RJ against acetic acid-induced colitis in rats (10). RJ includes polyphenolic compounds harvested by bees from the plants where they gather nectar (35). Polyphenolic compounds are the main components responsible for the functional properties of many foods, such as their antioxidant and anti-inflammatory capacity (36).
Conclusion
We observed immunosuppressive and immune-modulatory activities of RJ in the present study. We showed that treatment with RJ inhibited CD3-, CD5-, CD8- and CD45-positive T cells and decreased serum levels of TNF-α and IL-1β. In contrast, it increased serum levels of IL-10. However, these results require further confirmation with different colitis models and human studies.
Acknowledgement
This work was supported by grant no 2013/04 from the Trakya University Research Fund, Edirne, Turkey.
Footnotes
Conflict of interest
The authors declared that they have no competing interests.
References
- 1.Abboud PA, Hake PW, Burroughs TJ, Odoms K, O’Connor M, Mangeshkar P, et al. Therapeutic effect of epigallocatechin-3-gallate in a mouse model of colitis. Eur J Pharmacol. 2008;579:411–417. doi: 10.1016/j.ejphar.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 2.Safarpour AR, Hosseini SV, Mehrabani D. Epidemiology of inflammatory bowel diseases in Iran and Asia;A mini review. Iran J Med Sci. 2013;38:140–149. [PMC free article] [PubMed] [Google Scholar]
- 3.Carvalho AT, Souza H, Carneiro AJ, Castelo-Branco M, Madi K, Schanaider A, et al. Therapeutic and prophylactic thalidomide in TNBS-induced colitis: Synergistic effects on TNF-α, IL-12 and VEGF production. World J Gastroenterol. 2007;13:2166–2173. doi: 10.3748/wjg.v13.i15.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ko JK, Auyeung KK. Inflammatory bowel disease: etiology, pathogenesis and current therapy. Curr Pharm Des. 2014;20(7):1082–1096. doi: 10.2174/13816128113199990416. [DOI] [PubMed] [Google Scholar]
- 5.Mazzon E, Muia C, Di Paola R, Genovese T, De Sarro A, Cuzzocrea S. Thalidomide Treatment reduces colon injury induced by experimental colitis. Shock. 2005;23(6):556–564. [PubMed] [Google Scholar]
- 6.Prakash A, Medhi B, Avti PK, Saikia UN, Pandhi P, Khanduja KL. Effect of different doses of manuka honey in experimentally induced inflammatory bowel disease in rats. Phytother Res. 2008;22:1511–1519. doi: 10.1002/ptr.2523. [DOI] [PubMed] [Google Scholar]
- 7.Münstedt K, Bargello M, Hauenschild A. Royal jelly reduces the serum glucose levels in healthy subjects. J Med Food. 2009;12(5):1170–1172. doi: 10.1089/jmf.2008.0289. [DOI] [PubMed] [Google Scholar]
- 8.Viuda-Martos M, Ruiz-Navajas Y, Fernandez-Lopez J, Perez-Alvarez JA. Functional properties of honey, propolis, and royal jelly. J Food Sci. 2008;73(9):R117–R124. doi: 10.1111/j.1750-3841.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- 9.Kohno K, Okamoto I, Sano O, Arai N, Iwaki K, Ikeda M, et al. Royal jelly inhibits the production of proinflammatory cytokines by activated macrophages. Biosci Biotechnol Biochem. 2004;68:138–145. doi: 10.1271/bbb.68.138. [DOI] [PubMed] [Google Scholar]
- 10.Karaca T, Şimşek N, Uslu S, Kalkan Y, Can I, Kara A, et al. The effect of royal jelly on CD3(+), CD5(+), CD45(+) T-cell and CD68(+) cell distribution in the colon of rats with acetic acid-induced colitis. Allergol Immunopathol (Madr) 2012;40:357–361. doi: 10.1016/j.aller.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Gasic S, Vucevic D, Vasilijic S, Antunovic M, Chinou I, Colic M. Evaluation of the immunomodula-tory activities of royal jelly components in vitro. Immunopharmacol Immunotoxicol. 2007;29:521–536. doi: 10.1080/08923970701690977. [DOI] [PubMed] [Google Scholar]
- 12.Karaca T, Bayiroglu F, Yoruk M, Kaya MS, Uslu S, Comba B, et al. Effect of royal jelly on experimental colitis induced by acetic acid and alteration of mast cell distribution in the colon of rats. Eur J Histochem. 2010;54(4):e.35. doi: 10.4081/ejh.2010.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehrabani D, Bahrami F, Hosseini SV, Ashraf MJ, Tanideh N, Rezaianzadeh A, et al. The healing effect of Teucrium polium in acetic acid induced ulcerative colitis in the dog as an animal model. Middle East J Dig Dis. 2012;4:41–48. [PMC free article] [PubMed] [Google Scholar]
- 14.Tanideh N, Akbari Baseri F, Jamshidzadeh A, Ashraf MJ, Kuhi O, Mehrabani D. The healing effect of strawberry extract on acetic acid-induced ulcerative colitis in rat. World Appl Sci J. 2014;31(3):281–288. [Google Scholar]
- 15.Mazzon E, Muià C, Paola RD, Genovese T, Menegazzi M, De Sarro A, et al. Green tea polyphenol extract attenuates colon injury induced by experimental colitis. Free Radic Res. 2005;39(9):1017–1025. doi: 10.1080/10715760500197177. [DOI] [PubMed] [Google Scholar]
- 16.McCafferty DM, Sharkey KA, Wallace JL. Beneficial effects of local or systemic lidocaine in experimental colitis. Am J Physiol - Gas Liv Physiol. 1994;266:G560–G567. doi: 10.1152/ajpgi.1994.266.4.G560. [DOI] [PubMed] [Google Scholar]
- 17.Obermeier F, Kojouharoff G, Hans W, Schölmerich J, Gross V, Falk W. Interferon-gamma (IFN-γ)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin Exp Immunol. 1999;116:238–245. doi: 10.1046/j.1365-2249.1999.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keshavarz A, Minaiyan M, Ghannadi A, Mahzouni P. Effects of Carum carvi L. (Caraway) extract and essential oil on TNBS-induced colitis in rats. Res Pharm Sci. 2013;8:1–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Socca EA, Luiz-Ferreira A, de Faria FM, de Almeida AC, Dunder RJ, Manzo LP, Brito AR. Inhibition of tumor necrosis factor-alpha and cyclooxigenase-2 by Isatin: A molecular mechanism of protection against TNBSinduced colitis in rats. Chem Biol Interact. 2014;209:48–55. doi: 10.1016/j.cbi.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Paiotti AP, Miszputen SJ, Oshima CT, de Oliveira Costa H, Ribeiro DA, Franco M. Effect of COX-2 inhibitor after TNBS-induced colitis in Wistar rats. J Mol Histol. 2009;40:317–324. doi: 10.1007/s10735-009-9243-0. [DOI] [PubMed] [Google Scholar]
- 21.Bilsel Y, Bugra D, Yamaner S, Bulut Y, Cevikbas U, Turkoglu U. Could honey have a place in colitis therapy? Effects of honey, prednisolone, and disulfiram on inflammation, nitric oxide, and free radical formation. Dig Surg. 2002;19:306–311. doi: 10.1159/000064580. [DOI] [PubMed] [Google Scholar]
- 22.Oka H, Emori Y, Kobayashi N, Hayashi Y, Nomoto K. Suppression of allergic reactions by royal jelly in association with the restoration of macrophage function and the improvement of Th1/Th2 cell responses. Int Immunopharmacol. 2001;1:521–532. doi: 10.1016/s1567-5769(00)00007-2. [DOI] [PubMed] [Google Scholar]
- 23.Mehrabani D, Ziaei M, Hosseini SV, Ghahramani L, Bananzadeh AM, Ashraf MJ, et al. The effect of calendula officinalis in therapy of acetic acid induced ulcerative colitis in dog as an animal model. Iran Red Crescent Med J. 2011;13(12):884–890. [PMC free article] [PubMed] [Google Scholar]
- 24.Shen W, Durum S. Synergy of IL-23 and Th17 cytokines: New light on inflammatory bowel disease. Neurochem Res. 2010;35:940–946. doi: 10.1007/s11064-009-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, et al. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–2370. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 26.Fazio L, Cavazza E, Spisni E, Strillacci A, Centanni M, Candela M, et al. Longitudinal analysis of inflammation and microbiota dynamics in a model of mild chronic dextran sulfate sodium-induced colitis in mice. World J Gastroenterol. 2014;20:2051–2061. doi: 10.3748/wjg.v20.i8.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Celinski K, Dworzanski T, Fornal R, Korolczuk A, Madro A, Slomka M. Comparison of the anti-inflammatory and therapeutic actions of ppar-Gammaagonists rosiglitazone and troglitazone in experimental colitis. J Physiol Pharmacol. 2012;63:631–640. [PubMed] [Google Scholar]
- 28.Celinski K, Dworzanski T, Fornal R, Korolczuk A, Madro A, Slomka M. Comparsion of anti-inflammatory properties of peroxisome proliferator-activated receptor gammaagonists rosiglitazone and troglitazone in prophylactic treatment of experimental colitis. J Physiol Pharmacol. 2013;64:587–595. [PubMed] [Google Scholar]
- 29.Nagai T, Inoue R, Suzuki N, Nagashima T. Antioxidant properties of enzymatic hydrolysates from royal jelly. J Med Food. 2006;9:363–367. doi: 10.1089/jmf.2006.9.363. [DOI] [PubMed] [Google Scholar]
- 30.Peng X, Li X, Wang W, Li N, Ma J, Shen S. Expression of Th1/Th2 inflammatory cytokines in rat treatment model of ulcerative colitis (Abstract) J Central South Univ. 2013;38:1020–1028. doi: 10.3969/j.issn.1672-7347.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Wang K, Xuan X, Wang L, Tong L, Huang Q, Zhu L, et al. Expression and correlation analysis between inflammatory cytokines and calprotectin in the rat model of ulcerative colitis. Chinese J Cell Mol Immunol. 2014;30:278–280. [PubMed] [Google Scholar]
- 32.Fujii A, Kobayashi S, Kuboyama N, Furukawa Y, Kaneko Y, Ishihama S, et al. Augmentation of wound healing by royal jelly (RJ) in streptozotocin-diabetic rats. Jpn J Pharmacol. 1990;53:331–337. doi: 10.1254/jjp.53.331. [DOI] [PubMed] [Google Scholar]
- 33.Okamoto I, Taniguchi Y, Kunikata T, Kohno K, Iwaki K, Ikeda M, et al. Major royal jelly protein 3 modulates immune responses in vitro and in vivo. Life Sci J. 2003;73:2029–2045. doi: 10.1016/s0024-3205(03)00562-9. [DOI] [PubMed] [Google Scholar]
- 34.Vucevic D, Melliou E, Vasilijic S, Gasic S, Ivanovski P, Chinou I, et al. Fatty acids isolated from royal jelly modulate dendritic cell-mediated immune response in vitro. Int Immunopharmacol. 2007;7:1211–1220. doi: 10.1016/j.intimp.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Fiorani M, Accorsi A, Blasa M, Diamantini G, Piatti E. Flavonoids from Italian multifloral honeys reduce the extracellular ferricyanide in human red blood cells. J Agric Food Chem. 2006;54:8328–8334. doi: 10.1021/jf061602q. [DOI] [PubMed] [Google Scholar]
- 36.Mehrabani D, Almasi-Hashiani A, Moshfeghi K, Khedmati E. Survival rate and its predictors in colorectal cancer patients, Southern Iran. Middle East J Sci Res. 2012;12:1072–1077. [Google Scholar]


