Abstract
Objective(s):
P-glycoprotein (P-gp) is an efflux protein, the overexpression of which has been associated with multidrug resistance in various cancers. Although siRNA delivery to reverse P-gp expression may be promising for sensitizing of tumor cells to cytotoxic drugs, the therapeutic use of siRNA requires effective carriers that can deliver siRNA intracellularly with minimal toxicity on target cells. We investigated a special class of PEGylated lipid-based nanoparticles (NP), named nanolipoparticles (NLPs), for siRNA-mediated P-gp downregulation.
Materials and Methods:
NLPs were prepared based on low detergent dialysis method. After characterization, we evaluated the effect of NLPs on siRNA delivery, and P-gp downregulation compared to oligofectamine™ (OFA) in vitro and in vivo.
Results:
Our results showed a significant decrease in P-gp expression and subsequent enhancement of chemosensitivity to doxorubicin in vitro. Although the effectiveness of NLPs for in vitro siRNA delivery compared to OFA was limited, the results of in vivo studies showed noticeable effectiveness of NLPs for systemic siRNA delivery. siRNA delivery using NLPs could downregulate MDR1 in tumor cells more than 80%, while OFA had a reverse effect on MDR1 expression in vivo.
Conclusion:
The results indicated that the prepared NLPs could be suitable siRNA delivery systems for tumor therapy.
Keywords: Breast cancer, Gene therapy, Liposome, Multidrug resistance, siRNA delivery, Tumor targeting
Introduction
MDR1 is the gene that encodes P-glycoprotein (P-gp), and it’s over expression has been known as a mechanism of drug resistance in several cancer types such as breast cancer (1, 2). Due to the nonspecific toxic effects, there are very few chemical P-gp inhibitors that may be useful for clinical use (3). The discovery of RNA interference (RNAi) and the possibility of downregulation of P-gp by small interfering RNAs (siRNAs) provides new fields for further studies (4). Therapeutic application of siRNA requires developing efficient carriers that: 1) have high affinity for siRNA; 2) protect siRNA from degradation in vivo; 3) deliver siRNA to the target cells; 4) enhance cellular uptake and endosomal escape and finally 5) release siRNA inside the cell (5).
In general, these problems can be partly overcome by forming complexes of siRNAs with cationic lipids or cationic polymers. However, in the presence of plasma proteins, the aggregation of positively charged complexes results in rapid elimination by reticuloendothelial system (RES) (half-life< 5 min) (6, 7).
Based on these facts many scientists tried to design new formulations with better properties for in vivo experiments. The liposomal nanoparticles with the general name of NP (7) are one of these new formulations for nucleic acid delivery. NP with small particle size (<100 nm) and encapsulated nucleic acids in a polyethylene glycol (PEG) -shielded bilayer shell have better stability in vivo (half-life: 1-10 hr) (8) compared with traditional lipoplexes.
Nanolipoparticles (NLPs) as a member of NP group are PEGylated nanoliposomes designed by Li et al (8) in order to use in vivo gene delivery experiments. NLPs with small particle size (< 100 nm) and neutral charges have better stability in vivo and are suitable for in vitro and in vivo DNA delivery (7).
Furthermore for an efficient tumor therapy, enhanced permeability and retention (EPR) effect can be exploited as a passive targeting phenomenon. Abnormal leaky tumor vessels and lack of efficient lymphatic drainage in tumors provide opportunity for nanoparticles to accumulate in tumor tissue much more than normal tissues (9). Small size and proximity to neutral surface charge of NLPs by reducing mononuclear phagocyte system (MPS) uptake enhance their circulation time and provide more opportunity for the EPR effect and passive accumulation in the tumor area.
So far, there has been some success in P-gp knockdown by siRNA silencing; however, for clinical trial experiments, a systemic method of delivery of siRNA is needed (4). On the other hand, NLPs not only have shown high efficiency for gene delivery in vitro studies (7, 8), they also have a good potential for in vivo applications. Based on these facts, we exploited the PEGylated NLPs for MDR1 siRNA delivery.
In our previous study, NLPs containing MDR1 siRNA were prepared based on low concentration detergent dialysis method (10). Liposomal characterization showed NLPs with the small size of 80 to 90 nm and the surface charge close to neutral, which had no toxicity on the MCF7/ADR cells. The encapsulation efficiency of siRNA in the NLPs was more than 80%. These results emphasize that the NLPs containing siRNA can be good candidates for in vivo studies and tumor targeting. We also demonstrated that the fluorescent siRNAs can be localized in MCF7/ADR cells, and the presence of serum in cell culture medium did not have any effects on siRNA localization (10). Therefore, NLPs containing MDR1 siRNA are suitable for more studies on multidrug resistance reversal in cancer therapy.
In current study, we investigated the ability of NLPs containing MDR1 siRNA for multidrug resistance reversal in breast carcinoma, in vitro and in vivo. We evaluated the effects of NLP mediated MDR1 siRNA delivery on MDR1 gene downregulation and P-gp expression by real time RT-PCR and western blotting analysis, respectively. We also investigated chemosensitivity enhancement to doxorubicin in MCF-7/ADR cells. After initial in vitro studies, NLPs were tested for tumor therapy in vivo.
Materials and Methods
Materials
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethelene glycol)-2000] (mPEG 2000-DSPE), 1,2-Dioleoyl-3-Trimethylammonium-Propane (DOTAP), 1,2-Dioleoyl-sn-Glycero-3-Phospho-ethanolamine (DOPE) were from Avanti Polar Lipids (Alabaster, AL, USA). Octyl β-D-glucopyranoside (OG) and Tris (hydroxymethyl)aminomethane and Diethyl pyrocarbonate (DEPC) were purchased from Invitrogen™ (USA). Oligofectamine™ (OFA) transfection reagent was purchased from Invitrogen™ (USA). All other chemicals used were of molecular grade. Deionized water was used in all experiments and then treated with DEPC for siRNA containing samples.
siRNAs
The anti-MDR1-specific siRNA duplex (siM) homologous to nt 88 to 108 (sense strand 5’-GAA ACC AAC UGU CAG UGU AdTdT-3’) was previously designed against the MDR1-encoding mRNA consensus sequence (GenBank accession number no. NM_000927) (11). In addition, a biologically active siRNA 5’-CGU ACG CGG AAU ACU UCG AdTdT-3’ directed against the non-mammalian luciferase (GL-2) encoding mRNA (siC) was used as a negative control. Both siRNAs were commercially obtained from Dharmacon (Lafayette, CO, USA) in annealed form.
Preparation and characterization of NLPs containing siRNA
siRNA was encapsulated into NLPs by the detergent dialysis method and characterized as described before (10). Briefly, mPEG- DSPE/- DOTAP/DOPE were mixed at a molar ratio 10/50/40 in chloroform, dried by rotary evaporation and placed under a high vacuum for 2 hr. Then, the dried lipid film was hydrated in 1.47 ml of 28 mM OG in 5 mM Tris buffer (pH 8.0) for 30 min. 14881pmol siRNA was separately solubilized in 1.47 ml of 28 mM OG in 5 mM Tris buffer (pH 8.0). The latter solution was gently added into the lipid solution and vortexed for 30 sec. Following 10 min incubation at room temperature for spontaneous particle formation, the mixture was transferred to a Slide-A-Lyzer™ dialysis cassette (MWCO 10 K; Pierce, Rockford, IL, USA) and dialyzed against 1 L of 5 mM Tris buffer (pH 8.0) for 2 days at 4°C. For the in vitro and in vivo studies, the buffer was changed to150 mM NaCl, 5 mM Tris buffer (pH 8.0) by dialysis.
Cell lines and cell culture
Human breast carcinoma cell line MCF-7 and established classical multidrug-resistant, P-gp-positive derivative, MCF-7/ADR (from Lage’s laboratory stocks) were grown as described (10).
Cell transfection
For cell transfection with NLPs and OFA (as a positive control), MCF-7/ADR Cells (3 ×105/well) were plated in six-well plates. The cells were then transfected at 30 to 40% confluence (10, 11). Transfection was performed in OPTI-MEM1 (Invitrogen™, USA) without serum or antibiotics in a final volume of 1 ml. In the case of NLPs, final concentrations of siRNA were calculated based on siRNA concentration in the formulation (5 µM). After 4 hr incubation at 37 °C, FBS (10% final concentration) was added.
Quantitative real-time RT-PCR analysis of MDR1 mRNA
MCF-7/ADR cells were transfected with liposomes containing siM (At 25, 50 and 100 nM concentrations) or with liposomes containing siC (100 nM) and empty liposomes (At lipid concentrations equivalent to 100 nM loaded liposomes). Each experiment was performed in triplicate. Total RNA was extracted from MCF-7 parental line, untreated MCF-7/ADR and all transfected groups 24 and 48 hr after transfection with a RNA extraction kit (RNeasy mini kit, Qiagen, Germany) according to the manufacturer protocol, and purity and integrity of RNAs were tested by measuring A260/A280 and running on 1% agarose gel, respectively.
After synthesizing cDNA using SuperScript™ First-Strand Synthesis System for RT-PCR (Invitrogen™, GmbH), real time PCR was carried out using LightCycler® Fast Start DNA Master SYBR Green I and the LightCycler 1.5 instrument (Roche Diagnostics, Mannheim, Germany) as Mosaffa et al described (12). All reactions were performed in duplicate. The forward and reversed primer sequences for MDR1 and β-actin genes (as a housekeeping gene) were; Primer F MDR1: 5’-CCC ATC ATT GCA ATA GCA GG -3’; Primer R MDR1: 5’-TGT TCA AAC TTC TGC TCC TGA -3’ (amplification product of 158 bp); Primer F β-actin: 5’-TCA TGA AGT GTG ACG TGG ACA TC -3’ and Primer R β- actin 5’-CAG GAG GAG CAA TGA TCT TGA TCT-3’ (amplification product of 156 bp); (MWG GmbH,Germany). Primers were chosen according to previous studies (13, 14). Melting curve analysis was performed to analyze quality of primers and products. Expression of target genes was normalized against β-actin. ΔΔCT method was used to measure fold increase of genes in compare to control group.
Western blot analysis
The total protein contents of NLP siM and OFA siM transfected groups were extracted after 48, 72 and 96 hr. Protein extraction for control groups was carried out after 48 hr. To prepare cell lysate, the cell culture plate was placed on ice, cells were washed with 1 x PBS and mixed with P38 buffer (Tris-HCl (62.5 mM), SDS (2% W/V), glycerol (10% W/V), DTT (50 mM) in protease inhibitor pretreated nuclease-free water (half of the complete protease inhibitor cocktail tablet (Roche) dissolved in 25 ml of double distilled water). The protein concentration was determined using the Pierce BCA Protein Assay Kit (Pierce, Rockford, IL, USA) according to the manufacturer’s protocol. Then cellular levels of MDR1/P-gp, in different experimental groups were analysed by western blotting as Stege et al described (15). MDR1/P-gp proteins were detected using a mouse monoclonal antibody (mAbs) C219 (Alexis, San Diego, CA, USA). We used mouse mAb against actin (mAb 1501 R; Chemicon, Temecula, CA) as a loading control and peroxidase-conjugated goat anti- mouse IgG (A-1949; Sigma, St. Louis, MO, USA) as a secondary antibody. Finally, protein bands were analyzed using UVtec software (UK). Experiments performed in triplicate.
MTT assay for chemosensitivity enhancement determination
To evaluate the level of chemosensitivity enhancement following two days of siRNA transfection, MCF-7/ADR cells were incubated with 10 µM doxorubicin for 48 hr and the percentages of viable cells in different transfected groups was compared to untransfected control group as determined by MTT assay (16). Each assay was carried out in triplicate.
Animals for in vivo studies
Four-to-eight week-old female BALB/c mice were purchased from Pasteur Institute (Tehran, Iran). Mice were maintained in animal house of Pharmaceutical Research Center (MUMS, Iran) and fed with tap water and laboratory pellet chow (Khorassan Javane Co, Mashhad, Iran). Animals were housed in a colony room 12/12 hr light/dark cycle at 21 °C and had free access to water and food. All animal experiments were performed in compliance with the Institutional Ethical Committee and Research Advisory Committee of Mashhad University of Medical Sciences guidelines (Education Office, dated June 3, 2009; proposal code 87890).
Development of MCF-7/ADR tumor xenograft in mice
To achieve appropriate MCF-7/ADR xenograft tumor size in female BALB/c mice, in a pilot study, different immunosuppressors with different doses were studied (17-20). Finally, mice were immune-suppressed by intraperitoneal injection of 150 mg/kg cyclophosphamide (Baxter, Germany) on two consecutive days. Mice were inoculated subcuta-neously with 107 MCF-7/ADR cells on the third day. They received 25 μg of β-estradiol 17-valerate (Aburaihan Pharmaceutical co., Iran) (dissolved in 50 µl of sesame oil (Sigma, USA)), simultaneously. Tumor sizes were assessed every other day. When the tumor grew to approximately 5 to 7 mm in diameter, mice were divided into 5 groups, three mice/groups, including a normal saline treated group (NS), an NLP control siRNA treated group (NLP siC 1.6 mg/kg), two NLP MDR1 siRNA treated group (NLP siM 0.8 mg/kg and NLP siM 1.6 mg/kg), and an oligofectamine MDR1 siRNA treated group (OFA siM 1.6 mg/kg). All the mice were injected intravenously, and the volume of injection was adjusted to 200 µl.
Quantitative real-time RT-PCR for MDR1 mRNA detection in tumors
Tumor tissues were isolated 48 hr after siRNA administration and immediately frozen in liquid nitrogen for subsequent MDR1 mRNA level analysis. Total RNAs were extracted from all tumors using a tissue RNA extraction kit (High Pure RNA Tissue Kit, Roche) as described in the manufacturer’s protocol and real time PCR analysis carried out as described.
Statistical analysis
One-way ANOVA statistical test was used to assess the significance of the differences between the various groups. Tukey test was used to compare the means of different treatment groups in in vitro studies. In the case of in vivo studies, t test was used to compare each group with NS control group. P<0.05 was considered statistically significant.
Results
The effects of NLP siM on MDR1 mRNA expression
To assess multidrug resistance expression in MCF7/ADR cell line, we compared the levels of MDR1 mRNA expression in MCF7/ADR and its parental MCF-7. We observed that the levels of MDR1 mRNA in MCF7/ADR cells were about ten times more than its parental MCF-7 (Figure 1). At the next step, relative MDR1 mRNA expression was evaluated at 24 and 48 hr after transfection by real-time RT-PCR analysis. NLP siM and the positive control (OFA siM), significantly inhibited MDR1 expression in all concentrations at 24 and 48 hr (P<0.001) (Figure 2). Maximum MDR1 mRNA reductions were about 30.5% and 68.3% as obtained by NLP siM and OFA siM at 100 nM concentration after 48 hr, respectively. In the negative controls, although empty NLP had no significant effects on MDR1 expression, NLP siC reduced significantly MDR1 expression by 11.5% at 48 hr (P<0.05). Meanwhile, empty OFA and OFA siC significantly increased MDR1 mRNA expression by 14.5% and 19.6% (P<0.05) at 24 hr, respectively.
Figure 1.
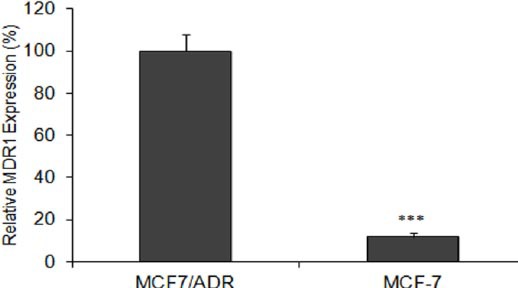
Relative mRNA levels for MDR1 in MCF-7/ADR human multidrug-resistant breast cancer cells and their parental cells MCF-7. The MDR1 mRNA expression value was normalized to β-actin. Data are means±SEM. (n = 3; *** P<0.001)
Figure 2.
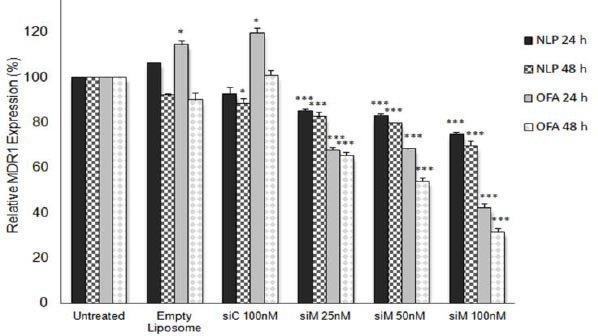
Relative mRNA levels for MDR1 in MCF-7/ADR human multidrug-resistant breast cancer cells. MCF-7/ADR Cells transfected with different formulations and incubated for 24 or 48 hr. MDR1 mRNA downregulation efficiency was calculated by comparing the MDR1 mRNA expression value in each transfected group to the untreated group. The MDR1 mRNA expression value was normalized to β-actin. Data are means±SEM. (n=3; *P<0.05, *** P<0.001)
NLP: Nanolipoparticle; OFA: Oligofectamine; siC: Control siRNA; siM: MDR1 siRNA
Downregulation of P-glycoprotein expression using NLP siM
For the evaluation of NLP siM efficacy in reversal of P-glycoprotein expression, western blotting was carried out on proteins extracted from different treated cells. MCF-7/ADR cells treated with NLP and OFA containing 100 nM siM or siC and with empty liposomes at equal concentration of lipid. Untreated MCF-7/ADR cells were used as the reference for resistant cells. As shown in Figure 3, significant decreases in P-gp expression (31.32 and 23.1%) were observed in NLP siM transfected cells after 72 and 96 hr (P<0.001), respectively. OFA siM 100 nM, positive control, could significantly reduce P-gp expression after 48 (P<0.01), 72 and 96 hr (P< 0.001) and maximum inhibition was obtained after 72 hr (57.7%), and remained nearly the same at 96 hr (53.3%). No significant effect observed in NLP siC and OFA siC treated groups. Interestingly, empty NLP and empty OFA significantly increased P-gp expression by 27.2% (P<0.001) and 30.9% (P<0.01), respectively.
Figure 3.
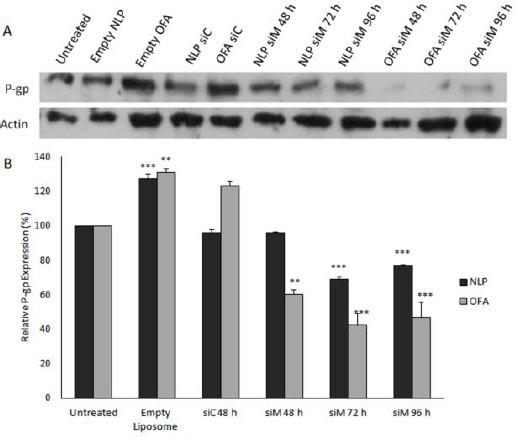
Western blot assay to determine the effect of MDR1 siRNA loaded nanoparticles on the P-gp expression. MCF-7/ADR Cells transfected with different formulations at the 100 nM and incubated for 48, 72 or 96 hr. P-gp downregulation efficiency was calculated by comparing the level of P-gp expression in each transfected group to the untreated group. (A) Top: P-glycoprotein was detected with C219 antibody. Bottom: The same membrane was reprobed with anti-actin antibody. (B) The quantitation of Western blot images carried out by using UVtec software and the protein levels were normalized against actin intensity. Data are means±SEM (n= 3; ** P<0.01, *** P<0.001).
NLP: Nanolipoparticle; OFA: Oligofectamine; siC: Control siRNA; siM: MDR1 siRNA
Chemosensitivity enhancement in MCF-7/ADR cells
MCF-7/ADR cells were pretreated with NLP siM 100 nM, empty NLP (as a negative control), NLP siC (as a negative control) and OFA siM 100 nM (as a positive control). Forty eight hours after transfection, cells were incubated with different concentrations of doxorubicin (1, 10 and 100 µM) for 24 and 48 hr. Treatment with 10 µM doxorubicin for 48 hr was chosen as an appropriate incubation time and concentration for maximum discrimination between liposome siRNA treated and untreated cells. Figure 4 shows the viability of different transfected MCF-7/ADR cells relative to the non-transfected control MCF-7/ADR cells after 48 hr incubation with 10 µM doxorubicin. The percentage of chemosensitivity enhancement was calculated as the decrease of cell viability percentage (Equation 2).
Figure 4.
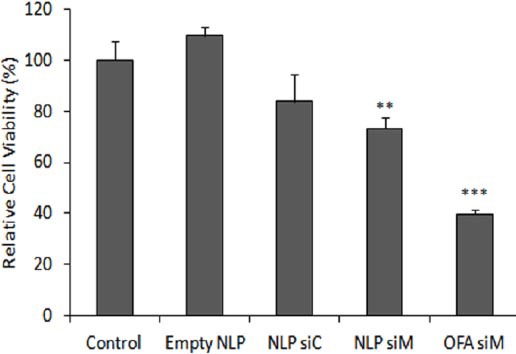
Enhancement of chemosensitivity to doxorubicin. Cytotoxicity of doxorubicin in MCF-7/ADR cells was assessed by MTT. Untransfected cells (control), cells transfected with liposomes containing MDR1 siRNA 100 nM (NLP siM, OFA siM) or cells transfected with empty NLP (NLP) or NLP containing control siRNA 100 nM (NLP siC) (as negative controls). After 48 hr, cells were incubated with 10 µM doxorubicin for 48 hr. Relative cell viability compared with control group has been reported. Data are means±SEM (n=3; ** P<0.01, *** P<0.001).
NLP: Nanolipoparticle; OFA: Oligofectamine; siC: Control siRNA; siM: MDR1 siRNA
Equation 2 chemosensitivity enhancement % = 100 - % cell viability.
NLP siM 100 nM led to significant 26.7% chemosensitivity enhancement (cell viability decrease to 73.3±4.1 (P< 0.01)), whereas OFA siM 100 nM as a positive control increased chemo-sensitivity to doxorubicin to 60.2% (cell viability decrease to 39.7±1.9% (P<0.001)). NLP siC 100 nM and empty NLP as a negative controls did not have any significant effects on chemosensitivity enhancement (P>0.05).
Downregulation of MDR1 mRNA expression in tumors
In order to confirm that siRNA may be delivered to the target cells in tumor tissue through systemic administration of NLPs, we assessed the effects of the siM on MDR1 gene expression in tumors. We determined NLP siM gene silencing efficiency in tumors (Figure 5). We found that NLP siM with the low dose of 0.8 mg/kg had no significant effect on MDR1 gene expression; however, by a dose increase to 1.6 mg/kg, significant gene silencing effect (80.9%±6.93 (P<0.001)) was observed. It is interes-ting that in control group, NlP siC, significantly enhanced MDR1 gene expression (about 82.5%±17.81 (P<0.01)). Similarly, in the case of OFA siM we observed significant MDR1 gene expression enhancing effect instead of gene silencing activity (342.3 ± 92.32 (P < 0.05)).
Figure 5.
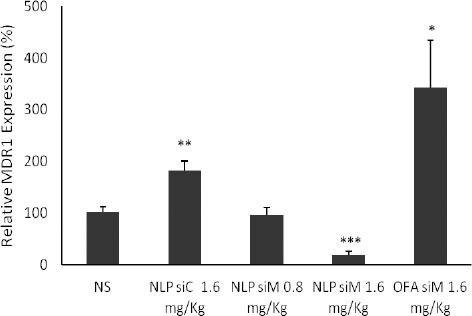
Functionality of nanolipoparticles containing MDR1 siRNA (NLP siM) and OFA siM in mice. 48 hr after systemic administration of formulated NLP siRNAs (0.8 and 1.6 mg/kg siM; 1.6 mg/kg siC) and OFA siM (1.6 mg/kg), tumor tissues harvested and total RNA was extracted for real-time RT-PCR analysis. The mRNA levels are shown relative to the control group (NS). The mRNA levels of MDR1 gene were normalized to the β-actin mRNA. Eeach group contained 3 mice and the reactions were performed in duplicate. The values are means ± SEM (*P<0.05, ** P<0.01, *** P<0.001)
NLP: Nanolipoparticle; siC: Control siRNA; siM: MDR1 siRNA; NS: Normal saline
Discussion
Non-toxic, suitable siRNA loaded NLPs with the stable size of 80 to 90 nm and the surface charge close to neutral (10). PEGylation by shielding positive surface charge of the particles and reducing their interactions enhance the stability and RES escape ability of the particles in vivo. Accomplishment of small size (less than 150 nm) with these characteristics results in circulation time enhancement and helps to exploit the EPR effect for passive targeting in cancer therapy (7). NLPs have all these characteristic properties making them suitable candidates for passive tumor targeting by EPR mechanism.
The effectiveness of MDR1 siRNA (siM) for P-gp downregulation was confirmed with oligofectamine™ (OFA) transfection. OFA as a good model of the commercial traditional liopoplexes has been frequently used for siRNA mediated P-gp downregulation (11). We evaluated MDR1 siRNA (siM) effects on P-gp downregulation in MCF-7/ADR cell line and compared siRNA delivery efficiency of NLP and OFA in vitro.
The assessment of MDR1 gene expression demonstrated that OFA siM and NLP siM even in low dose (25 nM) significantly reduced MDR1 gene expression at 24 and 48 hr. MDR1 gene silencing by NLP siM was accompanied by significant total P-gp downregulation at 72 and 96 hr. This 24 hr gap between mRNA and protein downregulation may have been resulted from P-gp long half-life (about 14-17 hr) (21). After 72 hr of treatment, the P-gp content started to increase again, and that can be explained by transient effects of siRNA. On the other hand, we observed significant MDR1 gene downregulation in NLP siC treated groups after 48 hr. This effect of NLP siC can be explained by off target effects of luciferase control siRNA. It has been reported that luciferase siRNA could remarkably increase or decrease the gene expression of about 2000 genes among 3300 genes in mammalian cells (22).
In order to confirm reversal of the MDR phenotype in MCF-7/ADR cells, chemosensitivity enhancement to doxorubicin analysis was used. NLP siM 100 nM significantly enhanced chemosensitivity to 10 µM doxorubicin in MCF-7/ADR cell line. Therefore, NLP siM could downregulate P-gp expression in vitro successfully, but its effects were less than OFA. High PEG in our formulation, 10% molar ratio, may be the cause for less efficiency of NLPs compared to OFA. PEG is a biocompatible and inert polymer, which can reduce interaction of nanoparticles with the cell membrane (23). The higher amounts of PEG present in our formulation might have reduced the delivery of siRNA in vitro. Nevertheless, PEGylation could considerably improve gene transfer and the safety of the delivery system in vivo via extension of circulation time, reduction of toxicity and stabilizing the particle PEG-lipid (24). It has been shown that increasing of mPEG-DSPE molar ratio in lipid DNA particles (LDPs) can reduce DNA delivery to B16/BL6 cells. It should be noted however, that this group did not test LDPs in vivo (25).
In order to overcome multidrug resistance in tumors via systemic siRNA administration, we developed an MCF-7/ADR xenograft tumor in BALB/c mice and tested the NLP formulation for siRNA silencing effects and compared the results to those of OFA in vivo. In the case of OFA siM and NLP siC control groups surprisingly; we observed the opposite effects on MDR1 expression as they increased MDR1 mRNA expression in vivo. These observations might be explained by two facts. First, it has been demonstrated that synthetic siRNA can act as a potent activator for innate immune system and induce a high level of IFN-α and inflammatory cytokines in mammalian cells, especially when carried by lipidic or policationic carriers (26, 27). Secondly, IFN-α can induce an increase in P-gp expression, that is accompanied by MDR1 mRNA increase (28). We suggest that NLP siC has no remarkable ability for MDR1 gene silencing, and OFA is not able to deliver the siM to tumors, Therefore, NLP siC and OFA siM could not silence the MDR1 expression efficiently, but might have up-regulated the MDR1 gene expression via innate immune stimulation. Furthermore, adding formulated liposomes to the cells may increase gene expression by unknown mechanisms (29). We observed the enhancement of MDR1 mRNA and P-gp expression after incubation with empty NLP, empty OFA and OFA siC treated cells in vitro. Since NLP siC in spite of OFA siC did not increase the MDR1 or P-gp expression in vitro, we can conclude that OFA compared to NLP may have had a more stimulating effect on cells.
In the case of NLP siM, although we could not see any significant effects at 0.8 mg/kg; however, intravenous administration of 1.6 mg/kg NLP siM could downregulate MDR1 mRNA more than 80%, while up to now just a limited number of groups could successfully downregulate P-gp via systemic administration of siRNA. For example, exploitation 2 and 4% PEGylated liposomes in MCF7/A xenograft tumors results in a significant P-gp downregulation (30). The P-gp downregulation was enhanced up to %60 by RGD modification of the PEGylated liposomes. This is while these liposomes have positive charge and their toxicity is more than NLPs toxicity. In another nonviral delivery approach, Patil et al (31) reduced the growth of JC breast cancer tumors in BALB/c mice to 50% with a single systemic injection of biotin-decorated poly(D,L-lactide-co-glycolide) nanoparticles.
Although a few studies have focused on systemic P-gp downregulation by nanoliposomes (30, 31), there are some reports of successful systemic siRNA administration by PEGylated nanoliposomes in animals (32, 33). It has been reported that siRNAs encapsulated in stable nucleic acid lipid particles (SNALP) remarkably downregulate target gene in non-human primates (33). They siRNA administered dose was 1 or 2.5 mg/kg wich results in more than 90% apolipoprotein B (APOB) gene silencing after 48 hr. In another attempt for systemic siRNA delivery, one research group could downregulate the expression of E6/7 oncogenes to around 50% in cervical cancer tumors. They exploited the hydration-of-freeze-dried-matrix (HFDM) method (34) to entrap siRNA within PEGylated liposomes. In these two studies, SNALP and HFDM-formulated particles contained 10% molar ratio PEG-S-DSG (C18) or PEG2000-C16 Ceramide. Although the presence of longer lipid anchors and higher PEG molar ratio in lipid based formulations could reduce cell interaction and transfection in vitro, they may increase tumor delivery because of more stability and more time in blood circulation. These theories may justify our findings. Existence of 10% mPEG-DSPE (C18) in NLP reduces in vitro cell transfection compared to non PEGylated liposomal OFA. However, systemic administration of NLPs containing MDR1 siRNA could downregulate significantly P-gp in tumor. These NLPs have small size and neutral surface charge; therefore, they have long circulation time in blood and can accumulate in tumor by EPR mechanism. Meanwhile, accumulation in tumor environment may provide enough time for NLPs to lose some of their PEG-lipids, and also due to the acidic pH of the tumor environment liposomes will be more positively charged compared to physiologic pH of 7.4, and therefore have more cell interaction compared to in vitro experiments.
Conclusion
In this study we prepared PEGylated nanoliposomes NLPs containing MDR1 siRNA based on detergent dialysis method. In order to investigate the ability of NLPs for siRNA delivery to tumors, the effects of NLP siM on MDR1 gene silencing were evaluated in vitro and in vivo. We observed that the NLPs delivered siRNA into MCF7/ADR cancer cells and tumors effectively. The NLP was more successful in delivery of siRNA into tumors compared to the traditional lipoplex, oligofectamine™.
In vivo anti-tumor activity of the NLP-siRNA in animal tumor model is under investigation. For further NLP siRNA delivery improvement, adding tumor targeting ligands or exchanging the PEGylated lipid to more removable PEG-lipids or pH-sensitive PEG-lipid can be exploited in future studies.
Acknowledgment
The authors are thankful to Dr A Derwiysh (Charite´ Campus Mitte, Institute of Pathology, Charite´platz 1, D-10117 Berlin, Germany) and Dr F Kalalinia (Biotechnology Research Center, Mashhad University of Medical Sciences, Mashhad, Iran) for their technical supports. This project was supported by a grant from Vice Chancellor for Research, Mashhad University of Medical Sciences, Mashhad, Iran. This report has been extracted from the PhD thesis of Mahnaz Nourbakhsh.
References
- 1.Mechetner E, Kyshtoobayeva A, Zonis S, Kim H, Stroup R, Garcia R, et al. Levels of multidrug resistance (MDR1) P-glycoprotein expression by human breast cancer correlate with in vitro resistance to taxol and doxorubicin. Clin Cancer Res. 1998;4:389–398. [PubMed] [Google Scholar]
- 2.Taheri M, Mahjoubi F, Omranipour R. Effect of MDR1 polymorphism on multidrug resistance expression in breast cancer patients. Genet Mol Res. 2010;9:34–40. doi: 10.4238/vol9-1gmr669. [DOI] [PubMed] [Google Scholar]
- 3.Liscovitch M, Lavie Y. Cancer multidrug resistance: a review of recent drug discovery research. IDrugs. 2002;5:349–355. [PubMed] [Google Scholar]
- 4.Abbasi M, Lavasanifar A, Uludaˇ H. Recent attempts at RNAi-mediated P-glycoprotein downregulation for reversal of multidrug resistance in cancer. Med Res Rev. 2013;33:33–53. doi: 10.1002/med.20244. [DOI] [PubMed] [Google Scholar]
- 5.Gao Y, Liu XL, Li XR. Research progress on siRNA delivery with nonviral carriers. Int J Nanomed. 2011;6:1017–1125. doi: 10.2147/IJN.S17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang L, Liu Y. In vivo delivery of RNAi with lipid-based nanoparticles. Annu Rev Biomed Eng. 2011;13:507–530. doi: 10.1146/annurev-bioeng-071910-124709. [DOI] [PubMed] [Google Scholar]
- 7.Li W, Szoka FC., Jr Lipid-based nanoparticles for nucleic acid delivery. Pharm Res. 2007;24:438–449. doi: 10.1007/s11095-006-9180-5. [DOI] [PubMed] [Google Scholar]
- 8.Li W, Huang Z, MacKay JA, Grube S, Szoka FC., Jr Low-pH-sensitive poly(ethylene glycol) (PEG)-stabilized plasmid nanolipoparticles: effects of PEG chain length, lipid composition and assembly conditions on gene delivery. J Gene Med. 2005;7:67–79. doi: 10.1002/jgm.634. [DOI] [PubMed] [Google Scholar]
- 9.Rastgoo M, Hosseinzadeh H, Alavizadeh H, Abbasi A, Ayati Z, Jaafari MR. Antitumor activity of PEGylated nanoliposomes containing crocin in mice bearing C26 colon carcinoma. Planta Med. 2013;79:447–451. doi: 10.1055/s-0032-1328363. [DOI] [PubMed] [Google Scholar]
- 10.Nourbakhsh M, Behravan J, Lage H, Abnous K, Mosaffa F, Badiee A, et al. Nanolipoparticles-mediated MDR1 siRNA delivery: preparation, characterization and cellular uptake. Nanomed J. 2015;2:39–45. [Google Scholar]
- 11.Stierle V, Laigle A, Jolles B. The reduction of P-glycoprotein expression by small interfering RNAs is improved in exponentially growing cells. Oligonucleotides. 2004;14:191–198. doi: 10.1089/oli.2004.14.191. [DOI] [PubMed] [Google Scholar]
- 12.Mosaffa F, Lage H, Afshari JT, Behravan J. Interleukin-1 beta and tumor necrosis factor-alpha increase ABCG2 expression in MCF-7 breast carcinoma cell line and its mitoxantrone-resistant derivative, MCF-7/MX. Inflamm Res. 2009;58:669–676. doi: 10.1007/s00011-009-0034-6. [DOI] [PubMed] [Google Scholar]
- 13.Kanzaki A, Takebayashi Y, Ren XQ, Miyashita H, Mori S, Akiyama S, et al. Overcoming multidrug drug resistance in P-glycoprotein/MDR1-overexpressing cell lines by ecteinascidin 743. Mol Cancer Ther. 2002;1:1327–1334. [PubMed] [Google Scholar]
- 14.Kalalinia F, Elahian F, Hassani M, Kasaeeian J, Behravan J. Phorbol ester TPA modulates chemoresistance in the drug sensitive breast cancer cell line MCF-7 by Inducing expression of drug efflux transporter ABCG2. Asian Pac J Cancer Prev. 2012;13:2979–2984. doi: 10.7314/apjcp.2012.13.6.2979. [DOI] [PubMed] [Google Scholar]
- 15.Stege A, Kruhn A, Lage H. Overcoming multidrug resistance by RNA interference. Methods Mol Biol. 2010;596:447–465. doi: 10.1007/978-1-60761-416-6_20. [DOI] [PubMed] [Google Scholar]
- 16.Ebrahimnejad P, Dinarvand R, Sajadi A, Jaafari MR, Nomani AR, Azizi E, et al. Preparation and in vitro evaluation of actively targetable nanoparticles for SN-38 delivery against HT-29 cell lines. Nanomed Nanotechnol Biol Med. 2010;6:478–485. doi: 10.1016/j.nano.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Hoogenhout J, Kazem I, Jerusalem CR, Bakkeren JA, de Jong J, Kal HB, et al. Growth pattern of tumor xenografts in Wistar rats after treatment with cyclophosphamide, total lymphoid irradiation and/or cyclosporin A. Int J Radiat Oncol Biol Phys. 1983;9:871–879. doi: 10.1016/0360-3016(83)90014-7. [DOI] [PubMed] [Google Scholar]
- 18.Fingert HJ, Chen Z, Mizrahi N, Gajewski WH, Bamberg MP, Kradin RL. Rapid growth of human cancer cells in a mouse model with fibrin clot subrenal capsule assay. Cancer Res. 1987;47:3824–3829. [PubMed] [Google Scholar]
- 19.Jalali SA, Sankian M, Tavakkol-Afshari J, Jaafari MR. Induction of tumor-specific immunity by multi-epitope rat HER2/neu-derived peptides encapsulated in LPD Nanoparticles. Nanomed Nanotechnol Biol Med. 2012;8:692–701. doi: 10.1016/j.nano.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Foldvari M, Jaafari MR, Radhi J, Segal D. Efficacy of the antiadhesin octyl O-(2-acetamido-2-deoxy-beta-D-galactopyranosyl)-(1-4)-2-O-propyl-beta-D-ga lactopy-ranoside (Fimbrigal-P) in a rat oral candidiasis model. Antimicrob Agents Chemother. 2005;49:2887–2894. doi: 10.1128/AAC.49.7.2887-2894.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller C, Laurent G, Ling V. P-glycoprotein stability is affected by serum deprivation and high cell density in multidrug-resistant cells. J Cell Physiol. 1995;163:538–544. doi: 10.1002/jcp.1041630314. [DOI] [PubMed] [Google Scholar]
- 22.Persengiev SP, Zhu X, Green MR. Nonspecific, concentration-dependent stimulation and repression of mammalian gene expression by small interfering RNAs (siRNAs) RNA. 2004;10:12–18. doi: 10.1261/rna5160904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wheeler JJ, Palmer L, Ossanlou M, MacLachlan I, Graham RW, Zhang YP, et al. Stabilized plasmid-lipid particles: construction and characterization. Gene Ther. 1999;6:271–281. doi: 10.1038/sj.gt.3300821. [DOI] [PubMed] [Google Scholar]
- 24.Fenske DB, MacLachlan I, Cullis PR. Stabilized plasmid-lipid particles: a systemic gene therapy vector. Methods Enzymol. 2002;346:36–71. doi: 10.1016/s0076-6879(02)46048-x. [DOI] [PubMed] [Google Scholar]
- 25.Harvie P, Wong FM, Bally MB. Use of poly(ethylene glycol)-lipid conjugates to regulate the surface attributes and transfection activity of lipid-DNA particles. J Pharm Sci. 2000;89:652–663. doi: 10.1002/(SICI)1520-6017(200005)89:5<652::AID-JPS11>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 26.Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S, et al. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005;11:263–270. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- 27.Sioud M. Induction of inflammatory cytokines and interferon responses by double-stranded and single-stranded siRNAs is sequence-dependent and requires endosomal localization. J Mol Biol. 2005;348:1079–1090. doi: 10.1016/j.jmb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Kang Y, Perry RR. Effect of α-interferon on p-glycoprotein expression and function and on verapamil modulation of doxorubicin resistance. Cancer Res. 1994;54:2952–2958. [PubMed] [Google Scholar]
- 29.Akhtar S, Benter I. Toxicogenomics of non-viral drug delivery systems for RNAi: Potential impact on siRNA-mediated gene silencing activity and specificity. Adv Drug Deliv Rev. 2007;59:164–182. doi: 10.1016/j.addr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Jiang J, Yang SJ, Wang JC, Yang LJ, Xu ZZ, Yang T, et al. Sequential treatment of drug-resistant tumors with RGD-modified liposomes containing siRNA or doxorubicin. Eur J Pharm Biopharm. 2010;76:170–178. doi: 10.1016/j.ejpb.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Patil YB, Swaminathan SK, Sadhukha T, Ma L, Panyam J. The use of nanoparticle-mediated targeted gene silencing and drug delivery to overcome tumor drug resistance. Biomaterials. 2010;31:358–365. doi: 10.1016/j.biomaterials.2009.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu SY, Singhania A, Burgess M, Putral LN, Kirkpatrick C, Davies NM, et al. Systemic delivery of E6/7 siRNA using novel lipidic particles and its application with cisplatin in cervical cancer mouse models. Gene Ther. 2011;18:14–22. doi: 10.1038/gt.2010.113. [DOI] [PubMed] [Google Scholar]
- 33.Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 34.Wu SY, Putral LN, Liang M, Chang HI, Davies NM, McMillan NA. Development of a novel method for formulating stable siRNA-loaded lipid particles for in vivo use. Pharm Res. 2009;26:512–522. doi: 10.1007/s11095-008-9766-1. [DOI] [PubMed] [Google Scholar]


