An uncontrolled immune system threatens human health as much as a defective one. Autoimmune diseases, such as type 1 diabetes (T1D), result from destruction of healthy tissues by a rogue immune system. Much of the self-policing of the immune system can be attributed to one subset of T cells called regulatory T cells (Tregs) (1). Patients with congenital defects in Tregs develop fatal multiorgan autoimmune diseases early in life (2). Tregs can be identified by the cell surface phenotype of CD4+CD25+CD127lo. CD25 is not merely a marker for Tregs but important for the maintenance and function of these cells as well. It associates with CD122 and CD132 to form the high-affinity receptor for interleukin (IL)-2, a growth factor for T cells. CD25 is constitutively expressed on Tregs, whereas its expression on other T cells is induced only after activation. Therefore, Tregs can better respond to IL-2 than other T cells in the steady state, prompting the consideration of using IL-2 therapeutically to expand Tregs for restoration of immune homeostasis.
One challenge to this approach is that other T cells and natural killer (NK) cells constitutively express CD122 and CD132 that can respond to high-dose IL-2, leading to their activation and tissue destruction. In fact, the current U.S. Food and Drug Administration–approved use of IL-2 is for enhancing immunity in patients with metastatic renal cell carcinoma and melanoma. Proleukin, a prescription form of IL-2, has an explicit warning for exacerbation of a variety of autoimmune and inflammatory diseases. In mouse models, high-dose IL-2 precipitated diabetes in a few days in prediabetic mice (3). As Tregs constitutively express high-affinity receptors for IL-2, low-dose IL-2, in theory, should preferentially boost Tregs without causing global immune activation. While this is shown to be true in mouse models (3,4), many issues remain in applying IL-2 therapy to humans. How low is low enough? Can a therapeutic window be defined for heterogeneous human populations? Will patients with autoimmune diabetes have a similar window? Are there biomarkers for IL-2 responsiveness so that personalized guidance for dose selection and therapeutic monitoring can be developed?
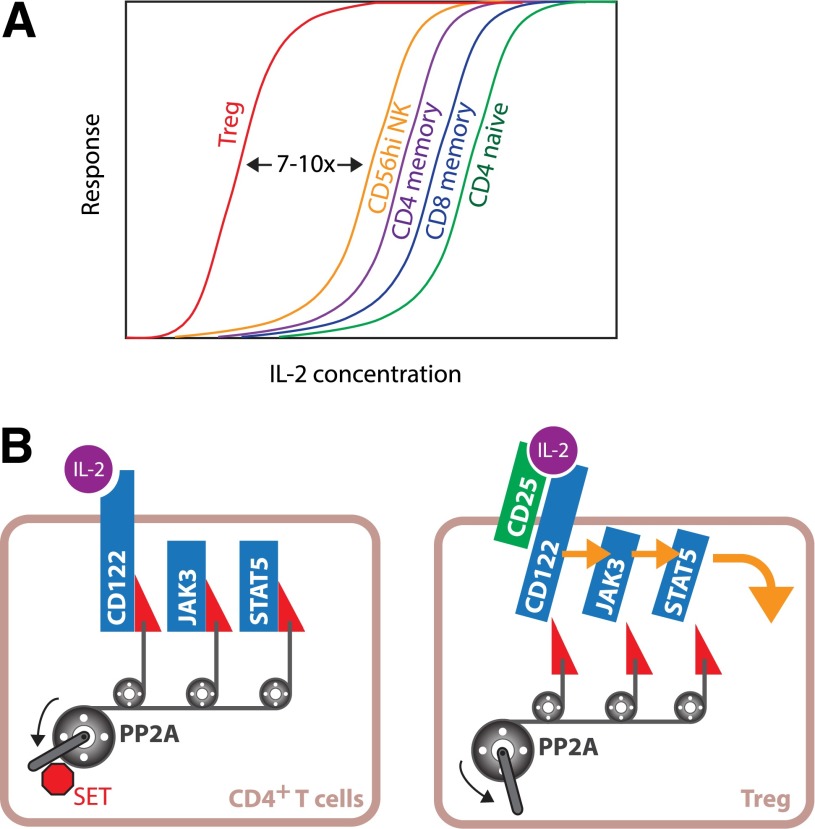
In this issue of Diabetes, Malek and colleagues (5) report their investigation of the cellular basis of Treg responsiveness to IL-2 and their findings help to address many of these crucial issues in applying IL-2 therapy for treatment of T1D. The investigators found that Tregs from normal individuals were invariably better responders to IL-2, followed by CD56hi NK cells and memory T cells (Fig. 1A). By calculating the half-maximal concentrations (EC50) for each cell type, the group was able to quantitatively measure the differences in IL-2 responsiveness and found that Tregs were 7 to 10 times more sensitive to IL-2 than NK cells and memory T cells. A new surprise was that the higher sensitivity of Tregs to IL-2 was not solely due to their higher expression of CD25. In vitro, activated CD4+ T cells expressed a higher level of CD25 but were still less responsive to IL-2 than Tregs. This suggests that factors in addition to CD25 expression contributed to higher sensitivity of Tregs. IL-2 signal transduction was mediated by JAK3 kinase and STAT5 (6). This pathway is counterregulated by multiple mechanisms; one of which is through serine/threonine phosphorylation of CD122, JAK3, and STAT5. Protein phosphatase 2A (PP2A), a serine/threonine phosphatase, releases the brake by dephosphorylating these proteins, thus enhancing IL-2 signaling (7). Malek and colleagues found that PP2A inhibition led to a greater decline of IL-2 responsiveness in Tregs than in other T-cell types, suggesting that Tregs had higher PP2A activity. This may be explained by the lower expression of the PP2A inhibitor protein, SET, in Tregs. Thus, Tregs are poised to respond to low-dose IL-2 because of their distinct intracellular wiring and constitutive expression of CD25 (Fig. 1B).
Figure 1.
High responsiveness of Tregs to IL-2 and its biochemical basis. A: Schematic diagram illustrating the hierarchy of IL-2 responsiveness of human peripheral blood lymphocytes. B: High sensitivity of Tregs to IL-2 can be explained by higher expression of CD25 and PP2A-dependent enhancement of intracellular IL-2 signal transduction.
The Malek team also investigated IL-2 responsiveness of Tregs from patients with T1D. A potential defect of IL-2 signaling in patients with T1D has been implicated by genome-wide association studies, and a previous study has shown impairment of IL-2–induced STAT5 phosphorylation in Tregs of patients with T1D (8,9). In contrast, the Malek team found that Tregs from subjects with T1D and normal subjects had similar EC50 of IL-2 induction of STAT5 phosphorylation. Moreover, the wide response gap between Tregs and CD4+ memory T cells was preserved in patients with T1D, which bolstered the team’s confidence that an IL-2 therapeutic window could be found for most patients with T1D.
It is worthwhile to note that the EC50 of IL-2 in Tregs was in the range of 2–5 pmol/L, which is approximately 1 IU/mL, 10 to 100 times lower than what is typically used in T-cell cultures. These new quantitative data suggest that the optimal dose for selective stimulation of Tregs using IL-2 may be much lower than current convention suggests. This is consistent with data emerging from early-stage clinical trials of IL-2 therapy in healthy volunteers and patients with graft-versus-host diseases, T1D, and hepatitis C virus–induced vasculitis. “Low-dose” IL-2 in the range of 1–3 × 106 IU/m2/day reliably expands Tregs but also leads to increases of CD56+ NK cells, the cell type that has the highest sensitivity to IL-2 after Tregs (10,11). Treg expansion is still observed after reducing IL-2 10 to 50 times to the “ultra–low-dose” range with less impact on NK cells (12–14).
Overall, this new report by Malek and colleagues (5) lends strong support for low-dose IL-2 therapy for boosting Tregs in patients with T1D. Although the investigators did not find a defect in IL-2 signaling among the small number of subjects with T1D that they analyzed, their gene expression array data clearly showed heterogeneity of Treg responsiveness to IL-2 among different individuals. Future study is needed to determine heterogeneity of responsiveness to low-dose IL-2 in Tregs as well as other IL-2–responsive cells, especially NK cells. In addition, group 2 innate lymphocytes constitutively express CD25 and mediate eosinophilia, one of the toxicities associated with IL-2 therapy (15). Understanding how group 2 innate lymphocytes respond to IL-2 will be important for defining the therapeutic window of IL-2.
Article Information
Funding. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK08231, to Q.T.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 2172.
References
- 1.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 2008;133:775–787 [DOI] [PubMed] [Google Scholar]
- 2.Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol 2003;15:430–435 [DOI] [PubMed] [Google Scholar]
- 3.Tang Q, Adams JY, Penaranda C, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity 2008;28:687–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grinberg-Bleyer Y, Baeyens A, You S, et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med 2010;207:1871–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu A, Snowhite I, Vendrame F, et al. . Selective IL-2 responsiveness of regulatory T cells through multiple intrinsic mechanisms supports the use of low-dose IL-2 therapy in type 1 diabetes. Diabetes 2015;64:2172–2183 [DOI] [PubMed] [Google Scholar]
- 6.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 2012;12:180–190 [DOI] [PubMed] [Google Scholar]
- 7.Ross JA, Cheng H, Nagy ZS, Frost JA, Kirken RA. Protein phosphatase 2A regulates interleukin-2 receptor complex formation and JAK3/STAT5 activation. J Biol Chem 2010;285:3582–3591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Todd JA, Walker NM, Cooper JD, et al.; Genetics of Type 1 Diabetes in Finland; Wellcome Trust Case Control Consortium . Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet 2007;39:857–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long SA, Cerosaletti K, Bollyky PL, et al. Defects in IL-2R signaling contribute to diminished maintenance of FOXP3 expression in CD4(+)CD25(+) regulatory T-cells of type 1 diabetic subjects. Diabetes 2010;59:407–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saadoun D, Rosenzwajg M, Joly F, et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med 2011;365:2067–2077 [DOI] [PubMed] [Google Scholar]
- 11.Koreth J, Matsuoka K, Kim HT, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med 2011;365:2055–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy-Nasser AA, Ku S, Castillo-Caro P, et al. Ultra low-dose IL-2 for GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation mediates expansion of regulatory T cells without diminishing antiviral and antileukemic activity. Clin Cancer Res 2014;20:2215–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito S, Bollard CM, Carlsten M, et al. Ultra-low dose interleukin-2 promotes immune-modulating function of regulatory T cells and natural killer cells in healthy volunteers. Mol Ther 2014;22:1388–1395 [DOI] [PMC free article] [PubMed]
- 14.Rosenzwajg M, Churlaud G, Mallone R, et al. Low-dose interleukin-2 fosters a dose-dependent regulatory T cell tuned milieu in T1D patients. J Autoimmun 2015;58:48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Gool F, Molofsky AB, Morar MM, et al. Interleukin-5-producing group 2 innate lymphoid cells control eosinophilia induced by interleukin-2 therapy. Blood 2014;124:3572–3576 [DOI] [PMC free article] [PubMed] [Google Scholar]