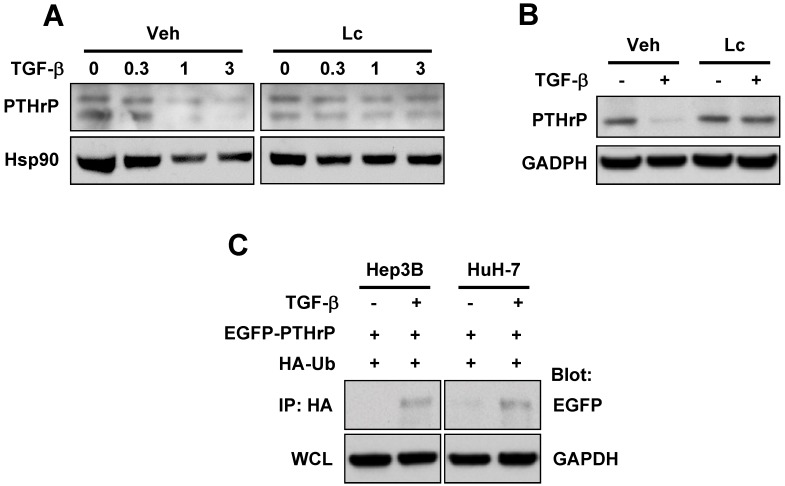
Figure 2.
TGF-β induces degradation of PTHrP through the ubiquitin-proteasome pathway. (A) Hep3B cells were serum-starved and treated for 48 h with different concentrations of TGF-β1 in the presence or absence of lactacystin (5 μM). Cell lysates were prepared and subjected to western blot analysis for PTHrP with Hsp90 expression as a loading control. (B) Serum-starved HuH-7 cells were treated with or without TGF-β1 (1 ng/ml) for 48 h and subjected to whole cell lysate preparation and western blot analysis. (C) Hep3B and HuH-7 cells were co-transfected with EGFP-tagged PTHrP (EGFP-PTHrP) and HA-tagged ubiquitin (HA-Ub). 72 h after transfection, the cells were serum-starved and treated with or without TGF-β1 (1 ng/ml) and subjected to whole lysate preparation and immunoprecipitation-western blot analysis.