Abstract
Objectives: Noise-induced hearing loss (NIHL) is an important occupational disease which results from an interaction between genetic and environmental factors. More and more evidences suggested that Catalase (CAT) gene polymorphism plays an important role in the development of NIHL. The aim of this study was to investigate the association of CAT gene polymorphisms with NIHL in a case-control study. Design: A total of 719 unrelated adult Chinese Han population, including 225 healthy volunteers and 494 noise-exposed workers were recruited in this study. Six tag single-nucleotide polymorphisms (tSNPs) were genotyped using an improved multiplex ligation detection reaction technique. Subsequently, the interaction between noise exposure level and genotypes and their effect on NIHL were analyzed using logistic regression. Results: Among six tSNPs, two of them (rs208679 and rs769217) were significantly associated with noise exposure level. For rs208679 recessive effect, GG genotype had a significantly increased of NIHL risk in the exposure level of < 85 dB; and for rs769217 dominant effect, the combined genotypes TT/TC had a significantly increased of NIHL risk in the exposure level of 85 dB~92 dB; and the haplotype A-G-T-C-A-C had a risk effect on the NIHL in the exposure level of 85 dB~92 dB. In addition, the rs769217 polymorphism could enhance the transcription activities of the CAT gene. Conclusions: This study identified CAT is a NIHL susceptibility gene when noise exposure levels are taken into account. Rs208679 and rs769217 polymorphisms might be used as relevant risk estimates for the development of NIHL in population with different noise exposure levels.
Keywords: Noise-induced hearing loss, tag, single-nucleotide polymorphism, catalase, clinical relevance
Introduction
Noise-induced hearing loss (NIHL) is the second most frequent forms of sensorineural hearing loss and forms of worldwide high occupational health-risk. It is mainly caused by continuous and regular exposure to noise, and also be the result of single or repeated occasions of acoustic trauma [1]. Recent studies suggest that NIHL is a complex multigenic disease, which has a large variation in susceptibility to noise, after exposure to the same duration and intensity of noise. NIHL is caused by an interaction between genetic and environmental factors. Noise, the best-known and the most studied environmental factors causing hearing loss, is harmful over 85 dB and causes both mechanical and metabolic damage [2]. Several other environmental factors, such as exposure to chemicals like organic solvents and heavy metals, also can augment the effect of noise [3,4]. In addition, some habit disturbances and disease such as smoking, high blood pressure and cholesterol levels can influence the susceptibility to noise [5]. Although it seems that susceptibility to noise is affected by genetic factors, up to now little is known about the genetic factors. No formal heritability studies regarding variability in noise susceptibility have been realized.
Oxidative stress is a biological condition that is characterized by production of excessive amounts of oxidants or decreased levels of antioxidants. There are numerous studies on the role of oxidative stress in hearing loss. It has been demonstrated that noise exposure can cause an initial increase in cochlear blood flow. Within a short period, there is an abrupt decrease in cochlear circulation seen by an aggregation of RBCs, capillary vasoconstriction, and stasis [6]. This intense metabolic activity and decreased cochlear blood flow from noise exposure alters cellular redox states and drives the formation of free radicals [7]. Excessive amounts of free radicals can damage cellular DNA, proteins, and lipids, as well as unregulated apoptotic pathways, causing cell death and irreparable damage to eloquent hearing structures [8]. On the other hand, oxidative stress results from impaired oxidative defense mechanisms, such as depletion of enzymatic (e.g., superoxide dismutase SOD, catalase CAT and glutathione peroxidase GPX) and non-enzymatic (e.g., glutathione GSH, vitamins A, C, and E, and selenium) antioxidants [9].
CAT is a ubiquitous enzyme found in all known organisms that is most abundant in liver, kidney, and erythrocytes. Together with SOD and GPX, CAT constitutes a primary defense against oxidative stress, functioning as an important regulator of H2O2 metabolism [10]. CAT gene polymorphism in promoter regions affects transcription factor binding and thus decreases CAT enzymatic activity leading to increased formation of hydroxyl radicals and elevated risk of diseases [11]. Recently, the association of the CAT gene polymorphisms with hypertension, angle closure glaucoma, Alzheimer’s disease and vitiligo has been investigated [12-14]. However, only scanty reports about the genetic effects on NIHL [6,15]. In addition, little is known about the global biosignificance of the entire genetic variants within the whole CAT gene.
Given the pivotal role of the CAT in the pathogenesis of NIHL, in this study, we hypothesized that the genetic variations in the CAT gene might affect the development of NIHL in workers following exposure to noise. To comprehensively assess the association of common genetic variants within the entire CAT gene with NIHL susceptibility, we selected a set of tag SNPs (tSNPs) to investigate their clinical relevance in relation to the development of NIHL in workers with noise exposure.
Materials and methods
Study design and data collection
A total of 719 unrelated Han Chinese adults, including 225 healthy volunteers and 494 factory workers with noise-exposure, were recruited from the Chongqing district for this study. The protocol of this study was approved by the Ethical and Protocol Review Committee of the Third Military Medical University. The benefits and risks of study participation were fully explained to each subject and written informed consent was obtained from the participants or their next-of-kin.
Healthy control group
The healthy volunteers control group comprised of 136 men and 89 women with a median age of 35 (18-55) years. Informed consent was obtained from the participants or their next commit university staff in the Daping hospital. Formal screening for psychological disorders was not undertaken in the control population. All controls were recruited in the Chongqing region and all were Han population.
Participants with noise-exposure
494 workers with noise-exposed were recruited from the factories where they were all exposed to at least 1 year of exposure to the continuous and steady noise in the regions of Fuling, Tongliang and Changshou in Chongqing, China, during the period from January 2011 to December 2012. Included workers had to meet the following criteria: Han Chinese, no history of middle ear disease, no family history of hearing loss, no history of using potentially ototoxic drugs (e.g., aspirin, quinolones, and amino glycosides), no history of fever or common infections (e.g., influenza, diarrhea, and hepatitis) within 1 month before medical examination. The health status was evaluated in all workers using a questionnaire and by clinical and laboratory examination. A questionnaire was used to obtain general information, occupational history, lifestyle (smoking and drinking status), history of explosive noise exposure, and history of disease [16]. The questionnaire was administered through face-to-face interviews by trained interviewer to obtain information. The clinical laboratory examination included signs, weight, height, pulse, electrocardiogram, B-echography, blood pressure, blood routine, and hepatic function test.
Audiological assessment and definitions of hearing loss
Pure-tone audiometry was performed for both ears of workers at 0.5, 1.0, 2.0, 3.0, 4.0, 6.0, and 8.0 kHz. All auditory tests were performed in a sound-attenuating booth by a trained technician. Audiometry was done using Swiss Midimate RT-150 Audiometer (BrT-1 & Kjaer Company) calibrated to ISO 389 (1985-E) for measurement of air conduction. Threshold value was defined as the lowest signal intensity that was detected in the subject at least 50% of the time, with a minimum of 3 tries. Masking was performed if the subject had a threshold value that differed by 40 dB or more between both ears. Otoscopic examination of the external acoustic meatus and tympanic membrane was done to exclude any ear diseases. Hearing loss can either be in the low-frequency range (0.5-2.0 kHz) or high-frequency range (4.0-8.0 kHz). We took the mean threshold of 0.5, 1.0, and 2.0 kHz (PTA512) as low-frequency hearing status and the mean threshold of 4.0, 6.0, and 8.0 kHz (PTA468) as high-frequency hearing status. Hearing threshold worse than 25 dB in either low frequency or high frequency was defined as hearing loss. A total of 191 unrelated Chongqing Han patients (115 men and 76 women) meeting diagnostic criteria for NIHL from 494 participants with noise exposure were recruited for the study.
Selections of SNPs
The full sequence of the human CAT gene (Accession Number: NC-000011.9 Reference GRCh37.p5 Primary Assembly) observed in the current study included 10 kb upstream of the transcription start site, all exons and introns and 10 kb downstream of the stop codon (33.235 kb total), which was pinpointed to chromosome 11, position 34460472-34493607 (data retrieved from Genbank in the Web site of NCBI). Genetic variation data for the entire CAT gene was obtained from the HapMap project for 45 healthy Chinese Han Beijing (CHB) populations (www.hapmap.org). From this database, a total of 105 SNPs were identified, among which, 44 SNPs with minor allele frequency (MAF) of more than or equal to 0.10 were selected for the analysis of htSNPs. Haplotype blocks were constructed throughout the entire CAT gene, using Haploview, version 4.2 (Broad Institute of MIT and Harvard, Cambridge, MA), a software package that provides computation of LD statistics and population haplotype patterns from genotype data [17]. Haplotype blocks represent regions inherited without substantial recombination in the ancestors of the current population. The history of recombination between a pair of SNPs can be estimated with the use of the normalized measure of allelic association D’ (value of D prime between the two loci) [18,19]. The criteria for the selected SNPs to construct a haplotype block is that all SNPs in one region must be in strong LD with D’ > 0.98 for the upper 95% confidence bound, and > 0.7 for the lower bound. A maximally informative htSNP was then selected from each block using software Tagger program (http://www.broad.mit.edu/mpg/haploview). This algorithm selects a subset of variants that capture all known common genetic variations in a gene, based on an LD threshold of r2 ≥ 0.8. The inverse of r2 represents the ratio of sample size needed to detect an indirect association with an un-assayed SNP to direct association at the same power.
SNPs genotyping
The genomic DNA was extracted from peripheral blood leukocytes using a Wizard Genomic DNA Purification Kit (Promega, Madison, Wisconsin, USA) following the manufacturer’s instructions. A multiplex polymerase chain reaction (PCR)-ligation detection reaction method was used for genotyping the selected 6 tag SNPs. For each SNP, the alleles were distinguished by different fluorescent labels of allele specific oligonucleotide probe pairs. Different SNPs were further distinguished by different extended lengths at the 3’ end. The primer information in two mixtures is described in (Table 1). Genotyping was performed in a blinded fashion without knowledge of the patients’ clinical data, and approximately 10% of the samples were genotyped in duplicate to monitor genotyping quality.
Table 1.
The primer sequence and concentration in PCR mixture1
| Primer Name | Primer Sequence | Annealing Temperature |
|---|---|---|
| rs10836233 F | TGCTATGGCCATCACCAGACAC | 54.6°C |
| rs10836233 R | CCACAGCTCCATGACTCCTGTTC | 56.5°C |
| rs208679 F | GCCCACTTTGTCACAGGCAGAA | 54.6°C |
| rs208679 R | GGCTCTACACTGAGGGATTTTCCAATA | 44.4°C |
| rs2300182 F | GTTGAAGCTTTCCTGCCCCACT | 54.6°C |
| rs2300182 R | CCTCCTGCCCAATGAAGGAATC | 54.6°C |
| rs7104301 F | TCAGCACTGATTTCACAACAGATCA | 48.0°C |
| rs7104301 R | TGGAGTCCTCGAGATACTGGCATTT | 49.0°C |
| rs769217 F | CCTTTTTGCCTATCCTGACACTCAC | 48.0°C |
| rs769217 R | AGGGGGAGCCCAACGTCTTTAG | 59.0°C |
| rs7949972 F | GCTGGTCTTTGGTTACCCTGGTATT | 48.0°C |
| rs7949972 R | CATTCCCCAGGGATCACTCTGA | 54.6°C |
Ex vivo CAT levels in whole blood
A human whole-blood assay was used centrifugation at 4,000 rpm for 15 min; the supernatants were aspirated and aliquoted for storage at -80°C. CAT in the supernatants was assayed with a sandwich enzyme linked immunosorbent assay (ELISA), according to the manufacturer’s instructions (Endogen, Woburn, MA, USA). The detection limits of the assay were 15 pg/ml.
Functionality of the rs769217 polymorphism
The possible effect of rs769217, which is located in exon9 of the CAT gene (Asp-389 T > C), on the transcription activity was investigated using a reporter gene assay system [20]. The DNA samples were amplified with a forward primer (5’ CAGCGTGACGGCCCGATGT 3’ for wild type, and 5’ CAGCGTGATGGCCCGAT GT 3’ for mutant type) and a reverse primer (5’ CCTCCAGGGCCACAGCAGAAC 3’). Accordingly, two different PCR products with 595 bp (from +22428 to +22923 of the CAT gene containing T or C allele at position +22436), including the rs769217-C and rs769217-T alleles respectively, were obtained, which is located in exo 5’ CAGCGTGACGGCCCGATGT 3’ for wild type, and 5’ CAGCGTGATGGCCCGAT GT 3’ for mutant type) and a reverse primer (5’ CCTCCAGGGCCACAGCAGAAC 3’). The PCR products had no sequence difference at any sites other than rs769217. The PCR products for each allele were gel purified by using MiniBEST Agarose Gel DNA Extraction Kit Ver.4.2 (TaKaRa, Dalian, China) and then cut with SacI and XhoI restriction enzymes. Two products were directly inserted into the SacI and XhoI sites of the pmirGlo du-luciferase reporter gene vector (Promega, Madison, WI, USA) by using T4 DNA ligase (TaKaRa, Dalian, China). The inserts containing rs769217-C or rs769217-T were cofirmed by PCR-direct sequencing. Plasmid with rs769217-C or rs769217-T was transfected into HEK293T cells using Lipofectamine 2000TM (Life Technologies, Carlsbad, CA, USA) and incubated for 48 h. The luciferase activities were determined by using the Dual-Glo Luciferase Assay system (Promega, Madison, WI, USA) with a luminometer. In this system, transcriptional activity was measured by Renilla luciferase activity and was normalized by Firefly luciferase activity in each assay (i.e., in the same well), results are expressed as fold increase in relative luciferase activity of Firefly and Renilla (F/R). The experiment was replicated three times, with quintuplicate assays performed within each replicate.
Statistical analyses
Allele frequencies for each tSNP were determined by gene counting. The genotype distribution of each tSNP was analyzed for deviations from the Hardy-Weinberg equilibrium using χ2 analyses. The extent of pairwise LD (r2-value) between polymorphisms was determined using Haploview version 4.2 software. Three genetic models were used (allele-dose, dominant, and recessive). A Fisher’s exact test was performed to identify statistical associations between genotype and disease status. A Pearson’s χ2 test was performed to identify statistical associations between allele and disease status. ORs with 95% confidence intervals were calculated by using multivariate logistic regression models to estimate the relative risk of NIHL. Haplotypes were generated and analyzed for linkage disequilibrium (LD) measures (D’ and r2) using JLIN [21]. All P-values were two-sided, and P < 0.05 after the Bonferroni correction for multiple testing was defined as statistically significant. All statistical analysis was carried out using the SPSS version 17.0 statistical software package (SPSS, Inc, Chicago, IL, USA).
Results
Construction of haplotype bins and selection of tag SNPs
There are a total of 6 tag SNPs with a minor allele frequency (≥ 10%) in the CHB population, which constructed four haplotype bins. Based on the analysis of tagging threshold of SNPs in each bin, one htSNP was selected for genotyping. With the pairwise analysis of linkage disequilibrium (LD) based on r2, we found there were 44 SNPs with a minor allele frequency (MAF) ≥ 10% within the CAT gene and the 10-kb up- and downstream regions. The selected six tag SNPs (tSNPs) are indicated by trigones. A LD plot of the 44 SNPs in the 33.235-kb region is displayed by using an r2 black-and-white color scheme. Black represents very high LD correlation between SNPs (r2 = 0.8 to 1), and white indicates the absence of correlation between SNPs (r2 = 0 to 0.2).
Allele frequencies and genotype distribution of the CAT gene polymorphisms
The genotyping success rates of the six tSNPs by an improved multiplex ligation detection reaction (iMLDR) technique ranged from 99.6% to 100% in our study cohort. The MAFs among the 225 healthy volunteers and the 494 workers with noise exposure were quite similar to those observed in the 45 unrelated CHB cohorts in the HapMap database. The genotype distribution of all six tSNPs was in agreement with the Hardy-Weinberg equilibrium (P > 0.05) (Tables 2, 3), indicating that the allele and genotype frequencies of these tSNPs in the population remain constant, which suggests that they are in equilibrium from generation to generation.
Table 2.
Frequencies of CAT gene polymorphisms in healthy volunteers and workers with noise exposure
| NCBI rsrs# | Location | Position | Variant | MAF (Hapmap-HCB) | healthy volunteers | workers with noise exposure | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Genotype, N | MAF | HWE | Genotype, N | MAF | HWE | |||||||||
| rs208679 | 5’flanking | -6411 | A/G | 0.22 | AA 130 | AG 82 | GG 13 | 0.24 | 0.99 | AA 285 | AG 179 | GG 30 | 0.24 | 0.79 |
| rs10836233 | 5’flanking | -2900 | G/A | 0.30 | GG 107 | GA 94 | AA 24 | 0.32 | 0.62 | GG 261 | GA 171 | AA 60 | 0.30 | 0.09 |
| rs2300182 | Intron1 | 7376 | T/A | 0.23 | TT 155 | TA 66 | AA 4 | 0.16 | 0.33 | TT 331 | TA 144 | AA 19 | 0.18 | 0.50 |
| rs769217 | exon9 (synon) | 22436 | T/C | 0.49 | TT 41 | TC 124 | CC 60 | 0.46 | 0.10 | TT 108 | TC 243 | CC 143 | 0.46 | 0.80 |
| rs7104301 | 3’flanking | 33166 | A/G | 0.23 | AA 124 | AG 85 | GG 16 | 0.26 | 0.98 | AA 279 | AG 184 | GG 31 | 0.25 | 0.93 |
| rs7949972 | 3’flanking | 41570 | C/T | 0.45 | CC 76 | CT 110 | TT 39 | 0.42 | 0.78 | CC 169 | CT 222 | TT 103 | 0.43 | 0.06 |
MAF indicates minor allele frequency; HWE indicates Hardy-Weinberg Equilibrium.
Table 3.
Frequencies of CAT gene polymorphisms in all workers without NIHL (-) and with NIHL (+)
| NCBI rs# | Location | Position | Variant | MAF (Hapmap-HCB) | without NIHL (-) | with NIHL (+) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Genotype, N | MAF | HWE | Genotype, N | MAF | HWE | |||||||||
| rs208679 | 5’flanking | -6411 | A/G | 0.22 | AA 171 | AG 112 | GG 20 | 0.25 | 0.77 | AA 114 | AG 67 | GG 10 | 0.23 | 0.97 |
| rs10836233 | 5’flanking | -2900 | G/A | 0.30 | GG 155 | GA 111 | AA 36 | 0.30 | 0.02 | GG 106 | GA 60 | AA 24 | 0.28 | 0.002 |
| rs2300182 | Intron1 | 7376 | T/A | 0.23 | TT 203 | TA 87 | AA 13 | 0.19 | 0.35 | TT 128 | TA 57 | AA 6 | 0.18 | 0.91 |
| rs769217 | exon9 (synon) | 22436 | T/C | 0.49 | TT 61 | TC 160 | CC 82 | 0.47 | 0.29 | TT 47 | TC 83 | CC 61 | 0.46 | 0.08 |
| rs7104301 | 3’flanking | 33166 | A/G | 0.23 | AA 173 | AG 115 | GG 15 | 0.24 | 0.46 | AA 106 | AG 69 | GG 16 | 0.26 | 0.32 |
| rs7949972 | 3’flanking | 41570 | C/T | 0.45 | CC 104 | CT 140 | TT 59 | 0.43 | 0.34 | CC 65 | CT 82 | TT 44 | 0.45 | 0.07 |
MAF indicates minor allele frequency; HWE indicates Hardy-Weinberg Equilibrium.
Clinical association of CAT gene polymophisms with development of NIHL in workers with noise exposure
A total of 719 unrelated Han Chinese adults, including 225 healthy volunteers and 494 workers with noise exposure, were recruited from the Chongqing district for this study. The worker cohort comprised a total of 494 consecutive Han Chinese patients, 303 males and 191 females, with mean ± SD age of 34.5 ± 5.4 years. Among these 494 workers with noise exposure, total of 191 unrelated Chongqing Han workers (121 men and 70 women) in accordance with the diagnostic criteria for NIHL. There were no significant differences in age and sex ratio among patients stratified according to the different genotypes of each tSNP. The P values of each polymorphism were analyzed with respect to a comparison between NIHL workers and the controls (including 225 healthy volunteers and the workers with noise exposure without NIH (-), respectively) by logistic analysis. It was found that none of six tag SNPs of the CAT gene has a significant effect on noise susceptibility across all exposure levels (P > 0.05). However we further used stratification analysis for the dominant or recessive effect and for the interaction between genotype and noise exposure level for every single SNP, we detected that in recessive effect, compared with the combined genotypes rs208679 GA/AA, rs208679 GG genotypes had a significantly increased effect on NIHL risk in the exposure level of < 85 dB (OR = 6.39, 95% CI: 0.83 to 49.35, P = 0.046) (Table 4); in dominant effect, compared with rs769217 CC genotypes, the combined genotypes rs208679 TT/TC had a significantly increased NIHL risk in the exposure level of 85 dB~92 dB (OR = 1.59, 95% CI: 1.02 to 2.48, P = 0.048) (Table 5) and healthy volunteers (OR = 1.62, 95% CI: 1.02 to 2.59, P = 0.043) (Table 6), which suggests a differential effect of the genotype on the noise susceptibility according to the noise exposure level.
Table 4.
Analyses of association of CAT gene polymorphisms with the risk of NIHL between workers with NIHL (+) and without NIHL (-) in the low-level exposure group (< 85 dB)
| NCBI rs# | Location | Variant | Genotype | Frequency | Allele-dose | Dominant* | Recessive# | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Without NIHL (-) | with NIHL (+) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | ||||
| rs208679 | 5’flanking | A/G | AA | 99 (58.20) | 42 (62.70) | 0.13 | 1.41 (0.86~2.30) | 0.56 | 1.21 (0.67~2.16) | 0.046 | 6.39 (0.83~49.35) |
| AG | 56 (32.90) | 24 (35.80) | |||||||||
| GG | 15 (8.80) | 1 (1.50) | |||||||||
| rs10836233 | 5’flanking | G/A | GG | 82 (48.50) | 35 (53.00) | 0.70 | 0.93 (0.60~1.44) | 0.82 | 1.16 (0.48~2.81) | 0.56 | 0.84 (0.47~1.48) |
| GA | 69 (40.80) | 23 (34.80) | |||||||||
| AA | 18 (10.70) | 8 (12.10) | |||||||||
| rs2300182 | Intron1 | T/A | TT | 111 (65.30) | 40 (59.70) | 0.67 | 1.25 (0.77~2.03) | 0.51 | 1.48 (0.42~2.23) | 0.46 | 1.27 (0.71~2.27) |
| TA | 52 (30.60) | 23 (34.30) | |||||||||
| AA | 7 (4.10) | 4 (6.00) | |||||||||
| rs769217 | exon9 (synon) | T/C | TT | 36 (21.20) | 16 (23.90) | 0.82 | 0.88 (0.59~1.32) | 1.00 | 0.96 (0.49~1.86) | 0.38 | 0.75 (0.38~1.45) |
| TC | 90 (52.90) | 36 (53.70) | |||||||||
| CC | 44 (25.90) | 15 (22.40) | |||||||||
| rs7104301 | 3’flanking | A/G | AA | 103 (60.60) | 37 (55.20) | 0.69 | 0.82 (0.51~1.30) | 0.47 | 0.80 (0.45~1.42) | 0.51 | 0.68 (0.19~2.39) |
| AG | 60 (35.30) | 26 (38.80) | |||||||||
| GG | 7 (4.10) | 4 (6.00) | |||||||||
| rs7949972 | 3’flanking | C/T | CC | 61 (35.90) | 19 (28.40) | 0.29 | 0.72 (0.48~1.08) | 0.23 | 0.68 (0.37~1.26) | 0.22 | 0.63 (0.33~1.23) |
| CT | 78 (45.90) | 30 (44.80) | |||||||||
| TT | 31 (18.20) | 18 (26.90) | |||||||||
Dominant effect = variant homozygotes + heterozygotes versus wild-type homozygotes;
Recessive effect = variant homozygotes versus heterozygotes + wild-type homozygotes.
Table 5.
Analyses of association of CAT gene polymorphisms with the risk of NIHL between workers with NIHL (+) and without NIHL (-) in the middle noise level exposure group (85~92 dB)
| NCBI rs# | Location | Variant | Genotype | Frequency | Allele-dose | Dominant* | Recessive# | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| without NIHL (-) | with NIHL (+) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | ||||
| rs208679 | 5’flanking | A/G | AA | 170 (56.40) | 72 (58.10) | 0.89 | 1.03 (0.73~1.25) | 0.83 | 1.07 (0.70~1.63) | 0.83 | 0.90 (0.40~2.04) |
| AG | 112 (37.00) | 43 (34.70) | |||||||||
| GG | 20 (6.60) | 9 (7.30) | |||||||||
| rs10836233 | 5’flanking | G/A | GG | 155 (51.30) | 71 (57.30) | 0.41 | 0.88 (0.64~1.23) | 0.29 | 0.79 (0.52~1.20) | 0.75 | 1.10 (0.58~2.06) |
| GA | 113 (37.40) | 38 (30.60) | |||||||||
| AA | 34 (11.30) | 15 (12.10) | |||||||||
| rs2300182 | Intron1 | T/A | TT | 203 (67.00) | 88 (71.00) | 0.36 | 0.79 (0.53~1.18) | 0.49 | 0.83 (0.53~1.31) | 0.25 | 0.37 (0.08~1.65) |
| TA | 87 (28.70) | 34 (27.40) | |||||||||
| AA | 13 (4.30) | 2 (1.60) | |||||||||
| rs769217 | exon9 (synon) | T/C | TT | 61 (20.10) | 31 (37.10) | 0.02 | 1.11 (0.82~1.49) | 0.048 | 1.59 (1.02~2.48) | 0.30 | 0.76 (0.46~1.24) |
| TC | 160 (52.80) | 47 (37.90) | |||||||||
| CC | 82 (27.10) | 46 (25.00) | |||||||||
| rs7104301 | 3’flanking | A/G | AA | 173 (57.10) | 69 (55.60) | 0.18 | 0.85 (0.61~1.19) | 0.83 | 0.94 (0.62~1.44) | 0.08 | 0.49 (0.22~1.07) |
| AG | 115 (38.00) | 43 (34.70) | |||||||||
| GG | 15 (5.00) | 12 (9.70) | |||||||||
| rs7949972 | 3’flanking | C/T | CC | 104 (34.30) | 46 (37.10) | 0.75 | 1.03 (0.76~1.38) | 0.58 | 1.16 (0.75~1.79) | 0.69 | 0.89 (0.53~1.50) |
| CT | 140 (46.20) | 52 (41.90) | |||||||||
| TT | 59 (19.50) | 26 (21.00) | |||||||||
Dominant effect = variant homozygotes + heterozygotes versus wild-type homozygotes;
Recessive effect = variant homozygotes versus heterozygotes + wild-type homozygotes.
Table 6.
Analyses of association of CAT gene polymorphisms with the risk of NIHL between healthy volunteers and workers with NIHL (+) in the middle noise level exposure group (85~92 dB)
| NCBI rs# | Location | Variant | Genotype | Frequency | Allele-dose | Dominant* | Recessive# | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| healthy volunteers | workers with NIHL (+) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | ||||
| rs208679 | 5’flanking | A/G | AA | 130 (57.80) | 72 (58.10) | 0.84 | 0.97 (0.67~1.39) | 1.00 | 1.01 (0.65~1.58) | 0.65 | 0.78 (0.33~1.89) |
| AG | 80 (36.40) | 43 (34.70) | |||||||||
| GG | 13 (5.80) | 9 (7.30) | |||||||||
| rs10836233 | 5’flanking | G/A | GG | 107 (47.60) | 71 (57.30) | 0.12 | 0.82 (0.58~1.15) | 0.60 | 1.24 (0.63~2.44) | 0.09 | 0.68 (0.44~1.05) |
| GA | 94 (41.80) | 38 (30.60) | |||||||||
| AA | 24 10.70) | 15 (12.10) | |||||||||
| rs2300182 | Intron1 | T/A | TT | 155 (68.90) | 88 (71.00) | 0.92 | 0.92 (0.60~1.41) | 0.72 | 0.91 (0.56~1.46) | 1.00 | 0.91 (0.16~5.02) |
| TA | 66 (29.30) | 34 (27.40) | |||||||||
| AA | 4 (1.80) | 2 (1.60) | |||||||||
| rs769217 | exon9 (synon) | T/C | TT | 41 (18.20) | 31 (37.10) | 0.009 | 1.08 (0.79~1.47) | 0.043 | 1.62 (1.02~2.59) | 0.17 | 0.67 (0.39~1.14) |
| TC | 124 (55.10) | 47 (37.90) | |||||||||
| CC | 60 (26.70) | 46 (25.00) | |||||||||
| rs7104301 | 3’flanking | A/G | AA | 124 (55.10) | 69 (55.60) | 0.65 | 0.95 (0.67~1.35) | 0.11 | 1.42 (0.93~2.17) | 0.42 | 0.72 (0.33~1.56) |
| AG | 85 (37.80) | 43 (34.70) | |||||||||
| GG | 16 (7.10) | 12 (9.70) | |||||||||
| rs7949972 | 3’flanking | C/T | CC | 76 (33.80) | 46 (37.10) | 0.44 | 0.99 (0.73~1.36) | 0.56 | 1.16 (0.73~1.83) | 0.47 | 0.79 (0.45~1.37) |
| CT | 110 (48.90) | 52 (41.90) | |||||||||
| TT | 39 (17.30) | 26 (21.00) | |||||||||
Dominant effect = variant homozygotes + heterozygotes versus wild-type homozygotes;
Recessive effect = variant homozygotes versus heterozygotes + wild-type homozygotes.
Combination effects of the six tag SNPs
Because LD has been suggested to be highly structured as conserved blocks of sequence separated by hotspots of recombination, the final function of a conserved haplotype may be the result of interaction among polymorphisms within the block. The LD coefficient between polymorphisms was calculated which showed that all genotyped polymorphisms are in strong LD. Six tagging SNPs in the LD block were elected, haplotypes were reconstructed and the haplotype frequencies were compared between workers without NIHL (-) and with NIHL (+). Haplotype analysis showed haplotype, ht3 (A-G-T-C-A-C), was significantly higher in workers with NIHL (+) compared with workers without NIHL (-) in the exposure level of 85 dB~92 dB, suggesting that haplotype ht3 had a risk effect on the NIHL development (OR = 2.27, 95% CI: 0.99 to 5.22, P = 0.048) (Table 7), but no significant effect on the exposure level of < 85 dB. However, haplotype association analysis did not provide a higher OR or a more significant result of P values than that of single locus SNPs.
Table 7.
Analyses of association of CAT gene haplotypes with the risk of NIHL between workers with NIHL (+) and without NIHL (-) in the middle noise level exposure group (85~92 dB)
| Loci | Genotype* | Frequency | Chi2 | Pearson’s p | Odds Ratio (95% CI) | |
|---|---|---|---|---|---|---|
|
| ||||||
| NIHL (+) | NIHL (-) | |||||
| ht1 | A A T T A C | 68.00 (0.27) | 168.99 (0.28) | 0.06 | 0.80 | 0.96 (0.69~1.3) |
| ht2 | A G A T A T | 37.99 (0.15) | 105.03 (0.17) | 0.64 | 0.43 | 0.85 (0.57~1.27) |
| ht3 | A G T C A C | 11.00 (0.04) | 11.98 (0.02) | 3.92 | 0.048 | 2.27 (0.99~5.22) |
| ht4 | A G T C G T | 64.01 (0.26) | 144.92 (0.24) | 0.23 | 0.63 | 1.09 (0.77~1.53) |
| ht5 | G G T C A C | 60.00 (0.24) | 149.98 (0.25) | 0.08 | 0.78 | 0.95 (0.67~1.35) |
Note: Haplotypes were ignored if the haplotype frequency was less than 3%.
Effect of rs208679 and rs769217 on CAT leels in whole blood
As shown in Figures 1 and 2, the CAT levels in whole blood were different among different genotypes. For the rs769217 polymorphisms, genotype CC (9.68 ± 4.12 ng/ml) > genotype CT (9.61 ± 3.09 ng/ml) > genotype TT (8.59 ± 3.96 ng/ml), however, there were no significant difference (P = 0.74). For the rs208679 polymorphisms, AA genotype: 9.08 ± 3.90 ng/ml, AG genotype: 9.79 ± 3.66 ng/ml and GG genotype: 10.58 ±1.27 ng/ml, which also showed no significant difference among different genotypes (P = 0.67).
Figure 1.
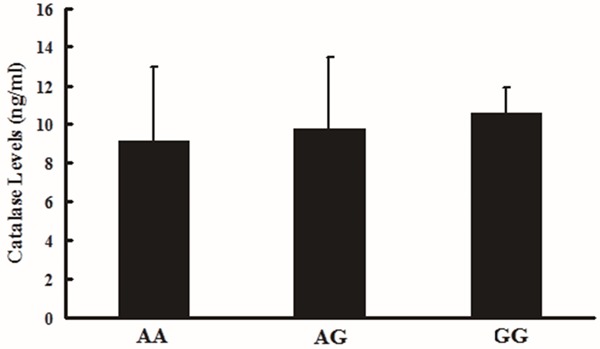
Effect of the rs208679 on human CAT blood levels (ng/ml). One-way ANOVA was used to assess statistical significance, AA (9.08 ± 3.90 ng/ml, n = 28), AG (9.79 ± 3.66 ng/ml, n=18), and GG (10.58 ±1.27 ng/ml, n=4), P = 0.67.
Figure 2.
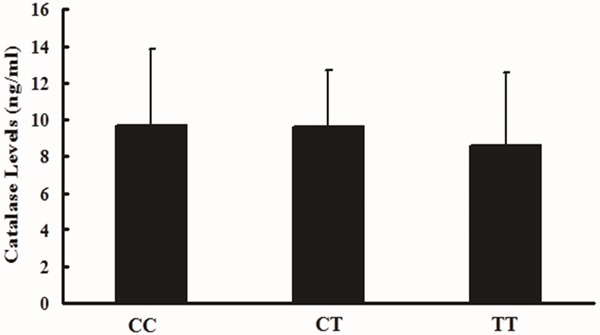
Effect of the rs769217 on human CAT blood levels (ng/ml). One-way ANOVA was used to assess statistical significance, CC (9.68 ± 4.12 ng/ml, n = 21), CT (9.61 ± 3.09 ng/ml, n=20), and TT (8.59 ± 3.96 ng/ml, n=9), P = 0.74.
Effect of the rs769217 on transcription activity
In view of the location of the rs769217 in the exon9 of the CAT gene (Asp-389 T>C), we further hypothesized that the T>C variation of this polymorphism might affect the transcription activities of the CAT gene. Figure 3 showed that the fold increase of RLA was significantly higher in cells transfected with wild C allele than those transfected with variant T allele (0.47 ± 0.005 vs. 0.50 ± 0.007, P = 0.004).
Figure 3.
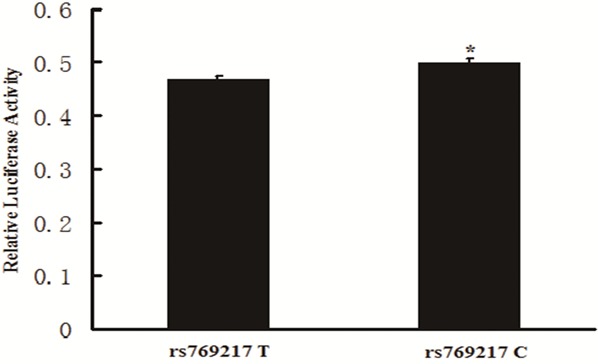
Effect of the rs769217 polymorphism of the CAT gene on the transcription activity. Relative luciferase activity (RLA) was measured in HEK293 cells transfected with rs769217C or rs769217T plasmid constructs as described in methods. The fold increase of RLA was significantly higher in cells transfected with wild C allele than those transfected with variant T allele (*P = 0.004 < 0.05 for rs769217C vs. 769217T).
Discussion
It has been demonstrated that inappropriate oxidative stress response contributes to the development of NIHL in workers with chronic noise exposure [22]. Increasing evidence suggests that genetic variants, particularly single nucleotide polymorphisms (SNPs), are critical determinants for inter-individual differences in susceptibility to NIHL [23]. CAT is an enzyme in the first line of antioxidant defense, which might contribute to the etiology and development of NIHL. In the present study, we want to identify the potential clinical relevance of the rs10836233, rs208679, rs2300182, rs769217, rs7104301 and rs7949972 polymorphisms, which are identified as tSNPs of the entire CAT gene in the Chinese Han population. tSNPs are a subset of all variants within a chromosome region of a disease study. The genetic effect of SNPs that are not genotyped in the study can be detected through LD with a tSNP. In this case, the six tSNPs selected in the current study theoretically reflect the biological significance of all the genetic variations across the entire CAT gene because of their strong LD compared with other un-assayed variants.
The association between six tSNPs in entire CAT gene and NIHL was analyzed. The results showed that none of six tag SNPs of the CAT gene has a significant effect on noise susceptibility across all exposure levels. However, the genotypes rs208679 GG had a significantly increased effect on NIHL risk in the exposure level of lower than 85 dB, and the combined genotypes rs769217 TT/TC had a significantly increased NIHL risk in the exposure level of 85~92 dB. Haplotype analysis showed haplotype, ht3 (A-G-T-C-A-C) was significantly higher in workers with NIHL (+) compared with without NIHL (-) in the exposure level of 85~92 dB, suggesting that haplotype ht3 had a risk effect on the NIHL development and no significant effect on the exposure level of < 85 dB. The results suggest that a significant difference existed in genotype and haplotype distribution between NIHL (+) and NIHL (-) workers for the different noise exposure groups.
Previous association study on the involvement of polymorphisms in oxidative stress genes in NIHL, including CAT, could not detect any significant association [24]. One report demonstrated that the effect of CAT polymorphisms of rs12273124, rs475046 and rs494024 on NIHL can be detected when noise exposure levels are taken into account in Sweden and Poland populations, while in our study the genotype frequencies of rs12273124, rs475046 and rs494024 in Chinese Han populations were 0%, 2% and 3% respectively (data not shown), were quite different from Sweden and Poland populations which all were greater than 10% [15]. These may be the result of ethnical difference.
The rs208679 polymorphism located in an internal promoter may affect expression levels of CAT, and rs769217 is a synonymous SNP in the exon9 of the CAT gene (Asp-389 T>C), it may be non-coding SNPs with an effect on the expression level or the activity of CAT. Therefore, we detected the CAT level in whole blood and the results suggested that both rs769217 and rs208679 can’t affect the expression level of CAT. Furthermore, using reporter gene assay system, we showed that the rs769217 polymorphism can affect the CAT transcription activity. It suggests that C > T variation at position Asp 389 Asp could significantly reduce the transcriptional activity of the CAT gene. Taken together, The C > T variation at position Asp 389 Asp might reduce DNA-protein interaction, and then inhibit CAT gene transcription leading to decreased risk of NIHL in workers with noise exposure.
Overall, the present study investigated the clinical relevance of the genetic variations within the entire CAT gene by means of construction of haplotype bins in workers with noise exposure. When subdividing the population by noise exposure level, and testing for association in the separate noise exposure strata, we demonstrated that rs769217 polymorphism showed a significant result in the high-level exposure group (85~92 dB) and rs208679 polymorphism showed a significant result in the low-level exposure group (< 85 dB). Whether this is the only NIHL susceptibility gene with this characteristic remains to be elucidated, as up to now only a limited number of studies on the genetic factors involved in NIHL have been performed in Chinese Han population. These results illustrate the complexity of NIHL and indicate that it is an interesting option to take into account of the exposure levels for future research that aims at the investigation of the genetic factors contributing to NIHL.
Acknowledgements
We acknowledge Dr. Ying Li, Li-Hua Nie, Jun-Wei Guo and Shan-Shan Gu for their clinical help, and Yi Ren, Rutgers, Prof. of the State University of New Jersey, for reviewing the article. And we also thank Shanghai Genesky Bio-Tech Genetic Core Lab for assistance in genotyping techniques. This work was supported by the National Natural Science Foundation of China (81000412).
Disclosure of conflict of interest
None.
References
- 1.Sliwinska-Kowalska M, Davis A. Noise-induced hearing loss. Noise Health. 2012;14:274–280. doi: 10.4103/1463-1741.104893. [DOI] [PubMed] [Google Scholar]
- 2.Lim DJ. Effects of noise and ototoxic drugs at the cellular level in the cochlea: a review. Am J Otolaryngol. 1986;7:73–99. doi: 10.1016/s0196-0709(86)80037-0. [DOI] [PubMed] [Google Scholar]
- 3.Fechter LD. Promotion of noise-induced hearing loss by chemical contaminants. J Toxicol Environ Health A. 2004;67:727–740. doi: 10.1080/15287390490428206. [DOI] [PubMed] [Google Scholar]
- 4.Sliwinska-Kowalska M, Zamyslowska-Szmytke E, Szymczak W, Kotylo P, Fiszer M, Wesolowski W, Pawlaczyk-Luszczynska M, Bak M, Gajda-Szadkowska A. Effects of coexposure to noise and mixture of organic solvents on hearing in dockyard workers. J Occup Environ Med. 2004;46:30–8. doi: 10.1097/01.jom.0000105912.29242.5b. [DOI] [PubMed] [Google Scholar]
- 5.Toppila E, Pyykko II, Starck J, Kaksonen R, Ishizaki H. Individual risk factors in the development of noise-induced hearing loss. Noise Health. 2000;2:59–70. [PubMed] [Google Scholar]
- 6.Quirk WS, Seidman MD. Cochlear vascular changes in response to loud noise. Am J Otol. 1995;16:322–325. [PubMed] [Google Scholar]
- 7.Evans P, Halliwell B. Free radicals and hearing: Cause, consequence, and criteria. Ann N Y Acad Sci. 1999;884:19–40. doi: 10.1111/j.1749-6632.1999.tb08633.x. [DOI] [PubMed] [Google Scholar]
- 8.Campbell KC, Meech R, Rybak L, Hughes L. The effect of D-methionine on cochlear oxidative state with and without cisplatin administration: mechanisms of otoprotection. J Am Acad Audiol. 2003;14:144–156. [PubMed] [Google Scholar]
- 9.Fetoni AR, Bartolo PD, Eramo SLM, Rolesi R, Paciello F, Bergamini C, Fato R, Paludetti G, Petrosini L, Troiani D. Noise-induced hearing loss (NIHL) as a target of oxidative stress-mediated damage: cochlear and cortical responses after an increase in antioxidant defense. J Neurosci. 2013;33:4011–4023. doi: 10.1523/JNEUROSCI.2282-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Labiós M, Martínez M, Gabriel F, Guiral V, Dasi F, Beltrán B, Muñoz A. Superoxide dismutase and catalase anti-oxidant activity in leucocyte lysates from hypertensive patients: effects of eprosartan treatment. J Renin Angiotensin Aldosterone Syst. 2009;10:24–30. doi: 10.1177/1470320309104067. [DOI] [PubMed] [Google Scholar]
- 11.Komina AV, Korostileva KA, Gyrylova SN, Belonogov RN, Ruksha TG. Interaction between single nucleotide polymorphism in catalase gene and catalase activity under the conditions of oxidative stress. Physiol Res. 2012;61:655–658. doi: 10.33549/physiolres.932333. [DOI] [PubMed] [Google Scholar]
- 12.Schallreuter KU, Salem MA, Gibbons NC, Martinez A, Slominski R, Ludemann J, Rokos H. Blunted epidermal L-tryptophan metabolism in vitiligo affects immune response and ROS scavenging by Fenton chemistry, part 1: epidermal H2O2/ONOO(-)- mediated stress abrogates tryptophan hydroxylase and dopa decarboxylase activities, leading to low serotonin and melatonin levels. FASEB J. 2012;26:2457–70. doi: 10.1096/fj.11-197137. [DOI] [PubMed] [Google Scholar]
- 13.Abu-Amero KK, Azad TA, Mousa A, Osman EA, Sultan T, Al-Obeidan SA. A catalase promoter variant rs1001179 is associated with visual acuity but not with primary angle closure glaucoma in Saudi patients. BMC Med Genet. 2013;14:84–90. doi: 10.1186/1471-2350-14-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bulut H, Pehlivan M, Alper S, Tomatir AG, Onay H, Yüksel SE, Özkinay F, Pehlivan S. Lack of association between catalase gene polymorphism (T/C exon9) and susceptibility to vitiligo in a Turkish population. Genet Mol Res. 2011;10:4126–4132. doi: 10.4238/2011.October.31.12. [DOI] [PubMed] [Google Scholar]
- 15.Konings A, van Laer L, Pawelczyk M, Carlsson P, Bondeson M, Rajkowska E, Dudarewicz A, Vandevelde A, Fransen E, Huyghe J. Association between variations in CAT and noise-induced hearing loss in two independent noise-exposed populations. Hum Mol Genet. 2007;16:1872–1883. doi: 10.1093/hmg/ddm135. [DOI] [PubMed] [Google Scholar]
- 16.Hu F, Li X, Li X, Wang M, Chu H, Liu K, Zhang H, Zhang Z, Zhu B. Lack of association between DNMT1 gene polymorphisms and noise-induced hearing loss in a Chinese population. Noise Health. 2013;5:231–236. doi: 10.4103/1463-1741.113517. [DOI] [PubMed] [Google Scholar]
- 17.Barrett JC, Fry B, Maller J. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 18.Gabriel SB, Schaffner SF, Nguyen H. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 19.Daly MJ, Rioux JD, Schaffner SF. High-resolution haplotype structure in the human genome. Nat Genet. 2001;29:229–232. doi: 10.1038/ng1001-229. [DOI] [PubMed] [Google Scholar]
- 20.Chorley BN, Wang XT, Campbell MR, Pittman GS, Noureddine MA, Bell DA. Discovery and verification of functional single nucleotide polymorphisms in regulatory genomic regions: current and developing technologies. Mutat Res. 2008;659:147–157. doi: 10.1016/j.mrrev.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carter KW, McCaskie PA, Palmer LJ. JLIN: a Java based linkage disequilibrium plotter. BMC Bioinformatics. 2006;7:60–68. doi: 10.1186/1471-2105-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seidman MD, Standring RT. Noise and quality of life. Int J Environ Res Public Health. 2010;7:3730–3738. doi: 10.3390/ijerph7103730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pawlaczyk-Luszczynska M, Dudarewicz A, Zaborowski K, Zamojska M, Sliwinska-Kowalska M. Noise induced hearing loss: research in central, eastern and south-eastern Europe and newly independent states. Noise Health. 2013;15:55–66. doi: 10.4103/1463-1741.107157. [DOI] [PubMed] [Google Scholar]
- 24.Carlsson PI, van Laer L, Borg E, Bondeson ML, Thys M, Fransen E, Van Camp G. The influence of genetic variation in oxidative stress genes on human noise susceptibility. Hear Res. 2005;202:87–96. doi: 10.1016/j.heares.2004.09.005. [DOI] [PubMed] [Google Scholar]


