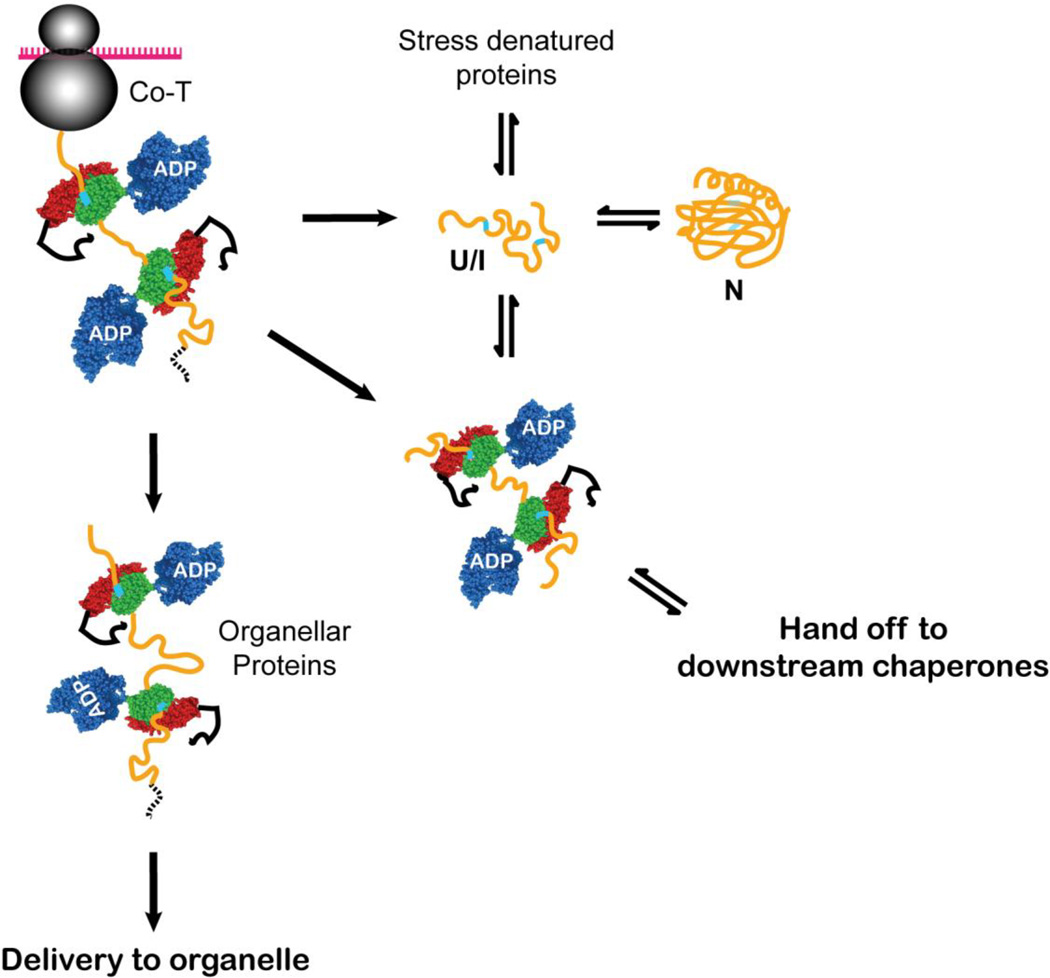
Figure 2.
Hsp70s function to maintain and favor the unfolded state of their substrates for a variety of downstream outcomes, including folding to the native state (N), delivery to organelles for translocation across membranes, and hand off to downstream chaperones (or degradation machinery in quality control pathways, not shown). The Hsp70 binding may occur co-translationally on a nascent chain or post-translationally to substrates that are released from the ribosome or stress-unfolded. Upon release from the Hsp70 interaction, the substrate is in an unfolded (U) or folding-competent intermediate (I) state. In this cartoon, the polypeptide substrate is shown in yellow with Hsp70 binding sites shown in cyan; an organellar targeting sequence is shown as a dashed black line. The Hsp70 subdomains are colored blue for the NBD, green for the β-subdomain of the SBD, red for the α-helical lid, and black for the unstructured C-terminal segment. In this and subsequent figures, the Hsp70 co-chaperones (J-proteins and nucleotide exchange factors) are not shown for simplicity.