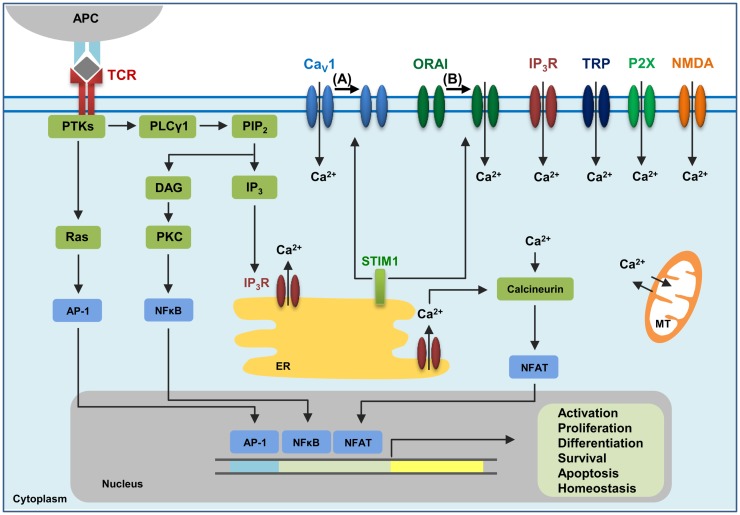
Figure 1.
The calcium channels in T cells. T cell receptor (TCR) engagement by a peptide-MHC on an antigen presenting cell (APC) induces protein tyrosine kinases (PTKs) to activate phospholipase Cγ1 (PLCγ1), which cleaves phosphatidylinositol 4,5-bisphosphate (PIP2) from plasma membrane phospholipids to generate diacylglycerol (DAG) and inositol-1,4,5-trisphosphate (IP3). Elevated levels of IP3 in the cytosol lead to the release of Ca2+ from IP3Rs located in the endoplasmic reticulum (ER). Ca2+ depletion from the ER induces Ca2+ influx from the extracellular space through the plasma membrane channel, ORAI1. Several additional channels also operate during TCR-mediated Ca2+ signaling. These include plasma membrane IP3R activated by the ligand IP3, transient receptor potential (TRP) channels that can be operated by DAG and SOCE, adenosine triphosphate (ATP)-responsive purinergic P2 (P2X) receptors, glutamate-mediated N-methyl-d-aspartate activated (NMDA) receptors, and voltage-dependent Ca2+ channels (CaV) that may be regulated through TCR signaling events. The mitochondria (MT) also control cytoplasmic Ca2+ levels. Increase in intracellular Ca2+ results in activation of calmodulin–calcineurin pathway that induces NFAT nuclear translocation and transcription of target genes to direct T cell homeostasis, activation, proliferation, differentiation, apoptosis and survival. Within this complex network of Ca2+ signaling, a model of the reciprocal regulation of CaV1 and ORAI1 in T cells has been proposed. (A) Low-level TCR signaling through interactions with self-antigens (i.e., self-peptides/self-MHC molecules) may result in CaV1 (particularly CaV1.4) activation and Ca2+ influx from outside the cell. This allows for filling of intracellular Ca2+ stores and initiation of a signaling cascade to activate a pro-survival program within the naïve T cell. STIM1 is not activated in this scenario and, consequently, ORAI1 remains closed. (B) Strong TCR signaling through engagement by a foreign peptide-MHC induces the downstream signaling events that result in ER Ca2+ store depletion and STIM1 accumulation in puncta in regions of the ER near the plasma membrane allowing interactions with Ca2+ channels. ORAI1 enhances STIM1 recruitment to the vicinity of CaV1 channels. Here, STIM1 can activate ORAI1 while inhibiting CaV1.